Page 130 - Chemistry ICSE Class IX
P. 130
5 The Periodic Table
Reasons for the Classification of Elements
CONTENTS
Before the nineteenth century, only a few elements were known. These
ͻ ZĞĂƐŽŶƐ ĨŽƌ ƚŚĞ ĐůĂƐƐŝĮĐĂƟŽŶ ŽĨ elements could be easily studied individually. With the passage of time,
ĞůĞŵĞŶƚƐ many more new elements were discovered. More and more of their
ͻ ĂƌůŝĞƌ ĂƩĞŵƉƚƐ ĨŽƌ ƚŚĞ ƉĞƌŝŽĚŝĐ
ĐůĂƐƐŝĮĐĂƟŽŶ ŽĨ ĞůĞŵĞŶƚƐ compounds were prepared. Study of these elements and compounds
ͻ DĞŶĚĞůĞĞǀΖƐ ƉĞƌŝŽĚŝĐ ůĂǁ individually became more dif cult. So, a need for proper classi cation of
ͻ DĞŶĚĞůĞĞǀΖƐ ƉĞƌŝŽĚŝĐ ƚĂďůĞ the elements into a few groups was felt.
ͻ DŽĚĞƌŶ ƉĞƌŝŽĚŝĐ ůĂǁ Certain reasons/objectives for the classi cation of elements were:
ͻ DŽĚĞƌŶ ůŽŶŐ ĨŽƌŵ ƉĞƌŝŽĚŝĐ ƚĂďůĞ To help study the elements and their compounds in a systematic and
ͻ WĞƌŝŽĚŝĐŝƚLJ ŝŶ ƉƌŽƉĞƌƟĞƐ organised manner.
ͻ ƚŽŵŝĐ ŶƵŵďĞƌ To correlate properties of the elements and their compounds with the
ͻ ƚŽŵŝĐ ƐŝnjĞ electronic con gurations of the elements.
ͻ sĂůĞŶĐĞ ĞůĞĐƚƌŽŶƐ To establish relationship between the behaviour of different elements.
ͻ sĂůĞŶĐLJ One of the earliest attempts towards the classi cation of elements was
ͻ dĞŶĚĞŶĐLJ ƚŽ ůŽƐĞ ĞůĞĐƚƌŽŶƐ to divide these into metals and nonmetals. This method of classi cation
ͻ dĞŶĚĞŶĐLJ ƚŽ ĂƩƌĂĐƚͬŐĂŝŶ ĞůĞĐƚƌŽŶƐ failed because most of the elements fell into the category of metals, whereas
ͻ DĞƚĂůůŝĐ ĂŶĚ ŶŽŶŵĞƚĂůůŝĐ ĐŚĂƌĂĐƚĞƌƐ only a few elements were nonmetals. There were a few elements which
ͻ ŚĞŵŝĐĂů ƌĞĂĐƟǀŝƚLJ showed the properties of both metals and nonmetals.
ͻ EĂƚƵƌĞ ŽĨ ŽdžŝĚĞƐ
ͻ hƐĞƐ ŽĨ ŵŽĚĞƌŶ ƉĞƌŝŽĚŝĐ ƚĂďůĞ Since then, many attempts were made by various scientists to classify
ͻ ůŬĂůŝ ŵĞƚĂůƐ ;'ƌŽƵƉ ϭͿ elements in a more systematic way. A few important attempts for the
ͻ ůŬĂůŝŶĞ ĞĂƌƚŚ ŵĞƚĂůƐ ;'ƌŽƵƉ ϮͿ classi cation of elements are described here.
ͻ ,ĂůŽŐĞŶƐ ;'ƌŽƵƉ ϭϳͿ
ͻ ĞƌŽ ŐƌŽƵƉ ;'ƌŽƵƉ ϭϴͿ Earlier Attempts for the Periodic Classification of
Elements
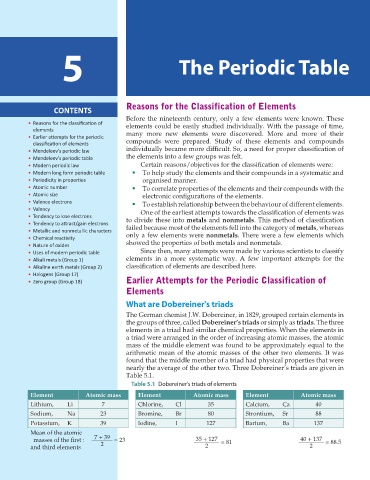
What are Dobereiner's triads
The German chemist J.W. Dobereiner, in 1829, grouped certain elements in
the groups of three, called Dobereiner’s triads or simply as triads. The three
elements in a triad had similar chemical properties. When the elements in
a triad were arranged in the order of increasing atomic masses, the atomic
mass of the middle element was found to be approximately equal to the
arithmetic mean of the atomic masses of the other two elements. It was
found that the middle member of a triad had physical properties that were
nearly the average of the other two. Three Dobereiner’s triads are given in
Table 5.1.
Table 5.1 Dobereiner's triads of elements
Element Atomic mass Element Atomic mass Element Atomic mass
Lithium, Li 7 Chlorine, Cl 35 Calcium, Ca 40
Sodium, Na 23 Bromine, Br 80 Strontium, Sr 88
Potassium, K 39 Iodine, I 127 Barium, Ba 137
Mean of the atomic
masses of the rst : 7 + 39 = 23 35 + 127 = 81 40 + 137 = 88.5
and third elements 2 2 2