Page 133 - Chemistry ICSE Class IX
P. 133
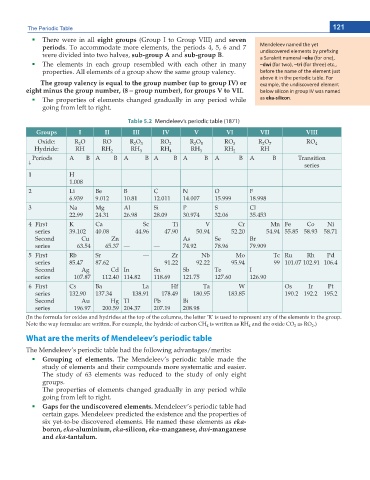
The Periodic Table 121
There were in all eight groups (Group I to Group VIII) and seven
periods. To accommodate more elements, the periods 4, 5, 6 and 7 DĞŶĚĞůĞĞǀ ŶĂŵĞĚ ƚŚĞ LJĞƚ
were divided into two halves, sub-group A and sub-group B. ƵŶĚŝƐĐŽǀĞƌĞĚ ĞůĞŵĞŶƚƐ ďLJ ƉƌĞĮdžŝŶŐ
Ă ^ĂŶƐŬƌŝƚ ŶƵŵĞƌĂů –eka ;ĨŽƌ ŽŶĞͿ͕
The elements in each group resembled with each other in many –dwi ;ĨŽƌ ƚǁŽͿ͕ –tri ;ĨŽƌ ƚŚƌĞĞͿ ĞƚĐ͕͘
properties. All elements of a group show the same group valency. ďĞĨŽƌĞ ƚŚĞ ŶĂŵĞ ŽĨ ƚŚĞ ĞůĞŵĞŶƚ ũƵƐƚ
ĂďŽǀĞ ŝƚ ŝŶ ƚŚĞ ƉĞƌŝŽĚŝĐ ƚĂďůĞ͘ &Žƌ
The group valency is equal to the group number (up to group IV) or ĞdžĂŵƉůĞ͕ ƚŚĞ ƵŶĚŝƐĐŽǀĞƌĞĚ ĞůĞŵĞŶƚ
eight minus the group number, (8 – group number), for groups V to VII. ďĞůŽǁ ƐŝůŝĐŽŶ ŝŶ ŐƌŽƵƉ /s ǁĂƐ ŶĂŵĞĚ
The properties of elements changed gradually in any period while ĂƐ eka-silicon͘
going from left to right.
Table 5.2 Mendeleev’s periodic table (1871)
Groups I II III IV V VI VII VIII
Oxide: R O RO R O 3 RO 2 R O 5 RO 3 R O 7 RO 4
2
2
2
2
Hydride: RH RH 2 RH 3 RH 4 RH 3 RH 2 RH
Periods A B A B A B A B A B A B A B Transition
p series
1 H
1.008
2 Li Be B C N O F
6.939 9.012 10.81 12.011 14.007 15.999 18.998
3 Na Mg Al Si P S Cl
22.99 24.31 26.98 28.09 30.974 32.06 35.453
4 First K Ca Sc Ti V Cr Mn Fe Co Ni
series 39.102 40.08 44.96 47.90 50.94 52.20 54.94 55.85 58.93 58.71
Second Cu Zn As Se Br
series 63.54 65.37 — — 74.92 78.96 79.909
5 First Rb Sr — Zr Nb Mo Tc Ru Rh Pd
series 85.47 87.62 91.22 92.22 95.94 99 101.07 102.91 106.4
Second Ag Cd In Sn Sb Te I
series 107.87 112.40 114.82 118.69 121.75 127.60 126.90
6 First Cs Ba La Hf Ta W Os Ir Pt
series 132.90 137.34 138.91 178.49 180.95 183.85 190.2 192.2 195.2
Second Au Hg Tl Pb Bi
series 196.97 200.59 204.37 207.19 208.98
(In the formula for oxides and hydrides at the top of the columns, the letter ‘R’ is used to represent any of the elements in the group.
Note the way formulae are written. For example, the hydride of carbon CH is written as RH and the oxide CO as RO .)
2
2
4
4
What are the merits of Mendeleev’s periodic table
The Mendeleev’s periodic table had the following advantages/merits:
Grouping of elements. The Mendeleev’s periodic table made the
study of elements and their compounds more systematic and easier.
The study of 63 elements was reduced to the study of only eight
groups.
The properties of elements changed gradually in any period while
going from left to right.
Gaps for the undiscovered elements. Mendeleev’s periodic table had
certain gaps. Mendeleev predicted the existence and the properties of
six yet-to-be discovered elements. He named these elements as eka-
boron, eka-aluminium, eka-silicon, eka-manganese, dwi-manganese
and eka-tantalum.