Page 137 - Chemistry ICSE Class IX
P. 137
The Periodic Table 125
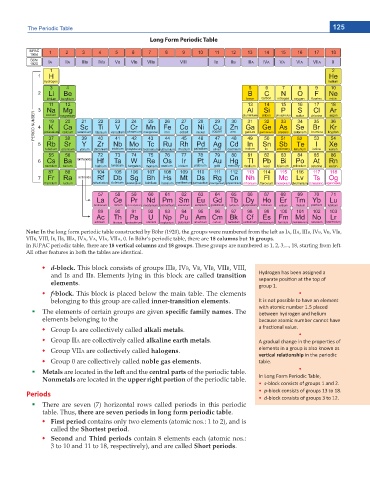
Long Form Periodic Table
Note: In the long form periodic table constructed by Böhr (1920), the groups were numbered from the left as IA, IIA, IIIB, IVB, VB, VIB,
VIIB, VIII, IB, IIB, IIIA, IVA, VA, VIA, VIIA, 0. In Böhr’s periodic table, there are 18 columns but 16 groups.
In IUPAC periodic table, there are 18 vertical columns and 18 groups. These groups are numbered as 1, 2, 3,..., 18, starting from left.
All other features in both the tables are identical.
d-block. This block consists of groups IIIB, IVB, VB, VIB, VIIB, VIII,
and IB and IIB. Elements lying in this block are called transition ,LJĚƌŽŐĞŶ ŚĂƐ ďĞĞŶ ĂƐƐŝŐŶĞĚ Ă
elements. ƐĞƉĂƌĂƚĞ ƉŽƐŝƟŽŶ Ăƚ ƚŚĞ ƚŽƉ ŽĨ
ŐƌŽƵƉ ϭ͘
f-block. This block is placed below the main table. The elements ͻ
belonging to this group are called inner-transition elements. /ƚ ŝƐ ŶŽƚ ƉŽƐƐŝďůĞ ƚŽ ŚĂǀĞ ĂŶ ĞůĞŵĞŶƚ
ǁŝƚŚ ĂƚŽŵŝĐ ŶƵŵďĞƌ ϭ͘ϱ ƉůĂĐĞĚ
The elements of certain groups are given URGEKſE HCOKN[ PCOGU. The ďĞƚǁĞĞŶ ŚLJĚƌŽŐĞŶ ĂŶĚ ŚĞůŝƵŵ
elements belonging to the ďĞĐĂƵƐĞ ĂƚŽŵŝĐ ŶƵŵďĞƌ ĐĂŶŶŽƚ ŚĂǀĞ
Group IA are collectively called alkali metals. Ă ĨƌĂĐƟŽŶĂů ǀĂůƵĞ͘
ͻ
Group IIA are collectively called alkaline earth metals. ŐƌĂĚƵĂů ĐŚĂŶŐĞ ŝŶ ƚŚĞ ƉƌŽƉĞƌƟĞƐ ŽĨ
Group VIIA are collectively called halogens. ĞůĞŵĞŶƚƐ ŝŶ Ă ŐƌŽƵƉ ŝƐ ĂůƐŽ ŬŶŽǁŶ ĂƐ
verƟcal relaƟonship ŝŶ ƚŚĞ ƉĞƌŝŽĚŝĐ
Group 0 are collectively called noble gas elements. ƚĂďůĞ͘
Metals are located in the left and the central parts of the periodic table. ͻ
Nonmetals are located in the upper right portion of the periodic table. /Ŷ >ŽŶŐ &Žƌŵ WĞƌŝŽĚŝĐ dĂďůĞ͕
ͻഩsͲďůŽĐŬ ĐŽŶƐŝƐƚƐ ŽĨ ŐƌŽƵƉƐ ϭ ĂŶĚ Ϯ͘
ͻഩpͲďůŽĐŬ ĐŽŶƐŝƐƚƐ ŽĨ ŐƌŽƵƉƐ ϭϯ ƚŽ ϭϴ͘
Periods
ͻഩdͲďůŽĐŬ ĐŽŶƐŝƐƚƐ ŽĨ ŐƌŽƵƉƐ ϯ ƚŽ ϭϮ͘
There are seven (7) horizontal rows called periods in this periodic
table. Thus, there are seven periods in long form periodic table.
First period contains only two elements (atomic nos.: 1 to 2), and is
called the Shortest period.
Second and Third periods contain 8 elements each (atomic nos.:
3 to 10 and 11 to 18, respectively), and are called Short periods.