Page 75 - Chemistry ICSE Class IX
P. 75
Water 63
ASSIGNMENT 1
Occurrence of water, Water - a chemical compound, Structure of water
ϭ͘ EĂŵĞ ƚŚĞ ĨŽƌŵƐ ŽĨ ǁĂƚĞƌ ĨŽƵŶĚ ŝŶ ŶĂƚƵƌĞ͘
Ϯ͘ ƌĂǁ ƚŚĞ ƐƚƌƵĐƚƵƌĞ ŽĨ ǁĂƚĞƌ ŵŽůĞĐƵůĞ͘
ϯ͘ EĂŵĞ ƚǁŽ ĐƌLJƐƚĂůůŝŶĞ ƐƵďƐƚĂŶĐĞƐ ŝŶ ǁŚŝĐŚ ǁĂƚĞƌ ŽĐĐƵƌƐ ĂƐ ĂƐƐŽĐŝĂƚĞĚ ǁĂƚĞƌ͘ 'ŝǀĞ ƚŚĞŝƌ ĐŚĞŵŝĐĂů ĨŽƌŵƵůĂĞ ĂůƐŽ͘
ϰ͘ tŚĂƚ ŵĂŬĞƐ ǁĂƚĞƌ ŵŽůĞĐƵůĞ ƉŽůĂƌ͍
ϱ͘ EĂŵĞ ƚŚĞ ƉƌŽƉĞƌƟĞƐ ƚŚĂƚ ŵĂŬĞ ǁĂƚĞƌ Ă ƵŶŝǀĞƌƐĂů ƐŽůǀĞŶƚ͘
Physical Properties of Water
What are the physical properties of water
Some physical properties of water are listed below:
Colour, taste, odour. Pure water is a colourless, odourless
and tasteless liquid.
Freezing and boiling points. Pure water freezes at 0°C and
boils at 100°C under 1 atm pressure.
When the pressure is increased, the boiling point of water
goes up and the freezing point goes down. When the
pressure is lowered, the boiling point of water decreases
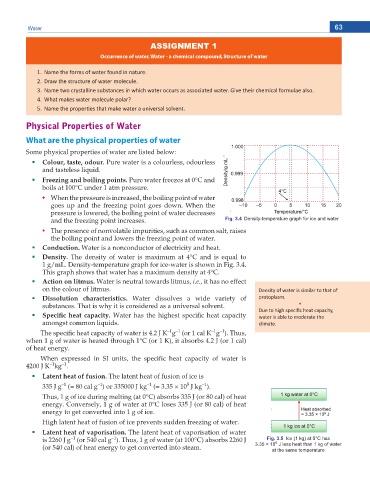
and the freezing point increases. Fig. 3.4 Density-temperature graph for ice and water
The presence of nonvolatile impurities, such as common salt, raises
the boiling point and lowers the freezing point of water.
Conduction. Water is a nonconductor of electricity and heat.
Density. The density of water is maximum at 4°C and is equal to
1 g/mL. Density-temperature graph for ice-water is shown in Fig. 3.4.
This graph shows that water has a maximum density at 4°C.
Action on litmus. Water is neutral towards litmus, i.e., it has no effect
on the colour of litmus. ĞŶƐŝƚLJ ŽĨ ǁĂƚĞƌ ŝƐ ƐŝŵŝůĂƌ ƚŽ ƚŚĂƚ ŽĨ
Dissolution characteristics. Water dissolves a wide variety of ƉƌŽƚŽƉůĂƐŵ͘
substances. That is why it is considered as a universal solvent. ͻ
ƵĞ ƚŽ ŚŝŐŚ ƐƉĞĐŝĮĐ ŚĞĂƚ ĐĂƉĂĐŝƚLJ͕
5RGEKſE JGCV ECRCEKV[ Water has the highest speci c heat capacity ǁĂƚĞƌ ŝƐ ĂďůĞ ƚŽ ŵŽĚĞƌĂƚĞ ƚŚĞ
amongst common liquids. ĐůŝŵĂƚĞ͘
–1 –1
–1 –1
The speci c heat capacity of water is 4.2 J K g (or 1 cal K g ). Thus,
when 1 g of water is heated through 1°C (or 1 K), it absorbs 4.2 J (or 1 cal)
of heat energy.
When expressed in SI units, the speci c heat capacity of water is
–1
–1
4200 J K kg .
Latent heat of fusion. The latent heat of fusion of ice is
–1
–1
–1
5
–1
335 J g (= 80 cal g ) or 335000 J kg (= 3.35 × 10 J kg ).
Thus, 1 g of ice during melting (at 0°C) absorbs 335 J (or 80 cal) of heat
energy. Conversely, 1 g of water at 0°C loses 335 J (or 80 cal) of heat
energy to get converted into 1 g of ice.
High latent heat of fusion of ice prevents sudden freezing of water.
Latent heat of vaporisation. The latent heat of vaporisation of water
–1
–1
is 2260 J g (or 540 cal g ). Thus, 1 g of water (at 100°C) absorbs 2260 J Fig. 3.5 Ice (1 kg) at 0°C has
5
(or 540 cal) of heat energy to get converted into steam. 3.35 × 10 J less heat than 1 kg of water
at the same temperature