Page 187 - Chemistry ICSE Class X
P. 187
Metallurgy 173
Most nonmetals are gases. Some nonmetals occur as solids. Bromine is
the only nonmetal which occurs as liquid under normal conditions.
Solid nonmetals — Carbon, Sulphur, Phosphorus, Iodine
Liquid nonmetal — Bromine
Gaseous nonmetals — Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine
What are metalloids
The elements which behave like metals as well as nonmetals are called
metalloids. Boron (B), Silicon (Si) and Arsenic (As) are metalloids.
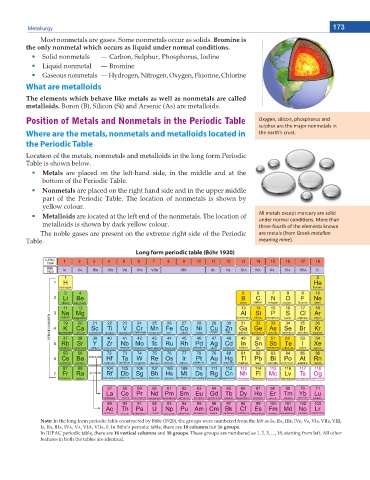
Position of Metals and Nonmetals in the Periodic Table KdžLJŐĞŶ͕ ƐŝůŝĐŽŶ͕ ƉŚŽƐƉŚŽƌƵƐ ĂŶĚ
ƐƵůƉŚƵƌ ĂƌĞ ƚŚĞ ŵĂũŽƌ ŶŽŶŵĞƚĂůƐ ŝŶ
Where are the metals, nonmetals and metalloids located in ƚŚĞ ĞĂƌƚŚ͛Ɛ ĐƌƵƐƚ͘
the Periodic Table
Location of the metals, nonmetals and metalloids in the long form Periodic
Table is shown below.
Metals are placed on the left-hand side, in the middle and at the
bottom of the Periodic Table.
Nonmetals are placed on the right hand side and in the upper middle
part of the Periodic Table. The location of nonmetals is shown by
yellow colour.
Metalloids are located at the left end of the nonmetals. The location of ůů ŵĞƚĂůƐ ĞdžĐĞƉƚ ŵĞƌĐƵƌLJ ĂƌĞ ƐŽůŝĚ
ƵŶĚĞƌ ŶŽƌŵĂů ĐŽŶĚŝƟŽŶƐ͘ DŽƌĞ ƚŚĂŶ
metalloids is shown by dark yellow colour. ƚŚƌĞĞͲĨŽƵƌƚŚ ŽĨ ƚŚĞ ĞůĞŵĞŶƚƐ ŬŶŽǁŶ
The noble gases are present on the extreme right side of the Periodic ĂƌĞ ŵĞƚĂůƐ ;ĨƌŽŵ 'ƌĞĞŬ metallon
Table. ŵĞĂŶŝŶŐ mineͿ͘
Long form periodic table (Böhr 1920)
Note: In the long form periodic table constructed by Böhr (1920), the groups were numbered from the left as IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIII,
IB, IIB, IIIA, IVA, VA, VIA, VIIA, 0. In Böhr’s periodic table, there are 18 columns but 16 groups.
In IUPAC periodic table, there are 18 vertical columns and 18 groups. These groups are numbered as 1, 2, 3, ..., 18, starting from left. All other
features in both the tables are identical.