Page 256 - Chemistry ICSE Class X
P. 256
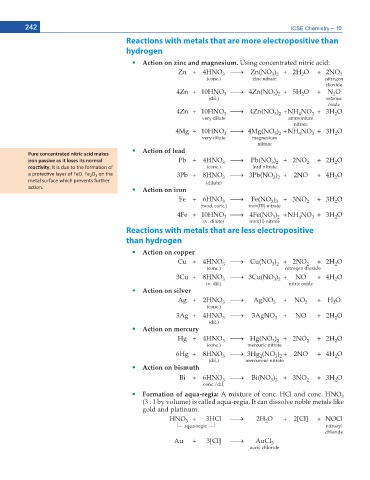
242 ICSE Chemistry – 10
Reactions with metals that are more electropositive than
hydrogen
Action on zinc and magnesium. Using concentrated nitric acid:
Zn + 4HNO o Zn(NO ) + 2H O + 2NO 2
3
2
3 2
(conc.) zinc nitrate nitrogen
dioxide
4Zn + 10HNO o 4Zn(NO ) + 5H O + N O
3 2
2
3
2
(dil.) nitrous
oxide
4Zn + 10HNO o 4Zn(NO ) + NH NO + 3H O
2
3 2
3
3
4
very dilute ammonium
nitrate
4Mg + 10HNO o 4Mg(NO ) + NH NO + 3H O
4
3 2
3
3
2
very dilute magnesium
nitrate
Action of lead
Pure concentrated nitric acid makes
iron passive as it loses its normal Pb + 4HNO o Pb(NO ) + 2NO + 2H O
2
2
3
3 2
ƌĞĂĐƟǀŝƚLJ͘ /ƚ ŝƐ ĚƵĞ ƚŽ ƚŚĞ ĨŽƌŵĂƟŽŶ ŽĨ (conc.) lead nitrate
Ă ƉƌŽƚĞĐƟǀĞ ůĂLJĞƌ ŽĨ &ĞK͘ &Ğ O ŽŶ ƚŚĞ 3Pb + 8HNO o 3Pb(NO ) + 2NO + 4H O
2 3
3 2
3
2
ŵĞƚĂů ƐƵƌĨĂĐĞ ǁŚŝĐŚ ƉƌĞǀĞŶƚƐ ĨƵƌƚŚĞƌ (dilute)
ĂĐƟŽŶ͘ Action on iron
Fe + 6HNO o Fe(NO ) + 3NO + 3H O
3 3
3
2
2
(mod. conc.) iron(III) nitrate
4Fe + 10HNO o 4Fe(NO ) + NH NO + 3H O
3
3 2
2
3
4
(v. dilute) iron(II) nitrate
Reactions with metals that are less electropositive
than hydrogen
Action on copper
Cu + 4HNO o Cu(NO ) + 2NO + 2H O
2
2
3 2
3
(conc.) nitrogen dioxide
3Cu + 8HNO o 3Cu(NO ) + NO + 4H O
2
3 2
3
(v. dil.) nitric oxide
Action on silver
Ag + 2HNO o AgNO + NO 2 + H O
3
2
3
(conc.)
3Ag + 4HNO o 3AgNO + NO + 2H O
2
3
3
(dil.)
Action on mercury
Hg + 4HNO o Hg(NO ) + 2NO + 2H O
3
2
2
3 2
(conc.) mercuric nitrate
6Hg + 8HNO o 3Hg (NO ) + 2NO + 4H O
3
2
3 2
2
(dil.) mercurous nitrate
Action on bismuth
Bi + 6HNO o Bi(NO ) + 3NO + 3H O
2
3
3 3
2
conc./dil.
Formation of aqua-regia: A mixture of conc. HCl and conc. HNO
3
(3 : 1 by volume) is called aqua-regia. It can dissolve noble metals like
gold and platinum.
HNO + 3HCl o 2H O + 2[Cl] + NOCl
2
3
aqua-regia nitrosyl
chloride
Au + 3[Cl] o AuCl 3
auric chloride