Page 60 - Chemistry ICSE Class X
P. 60
48 ICSE Chemistry – 10
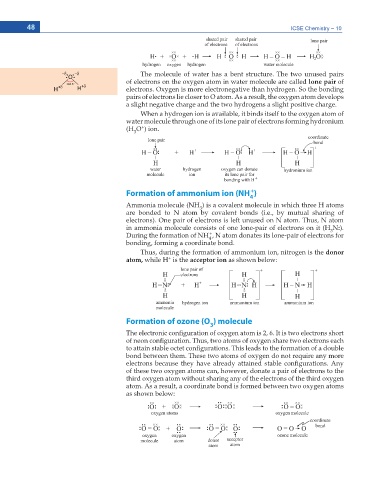
The molecule of water has a bent structure. The two unused pairs
of electrons on the oxygen atom in water molecule are called lone pair of
electrons. Oxygen is more electronegative than hydrogen. So the bonding
pairs of electrons lie closer to O atom. As a result, the oxygen atom develops
a slight negative charge and the two hydrogens a slight positive charge.
When a hydrogen ion is available, it binds itself to the oxygen atom of
water molecule through one of its lone pair of electrons forming hydronium
+
(H O ) ion.
3
+
Formation of ammonium ion (NH )
4
Ammonia molecule (NH ) is a covalent molecule in which three H atoms
3
are bonded to N atom by covalent bonds (i.e., by mutual sharing of
electrons). One pair of electrons is left unused on N atom. Thus, N atom
in ammonia molecule consists of one lone-pair of electrons on it (H N:).
3
+
During the formation of NH , N atom donates its lone-pair of electrons for
4
bonding, forming a coordinate bond.
Thus, during the formation of ammonium ion, nitrogen is the donor
+
CVQO while H is the acceptor ion CU UJQYP DGNQY
Formation of ozone (O ) molecule
3
6JG GNGEVTQPKE EQPſIWTCVKQP QH QZ[IGP CVQO KU +V KU VYQ GNGEVTQPU UJQTV
QH PGQP EQPſIWTCVKQP 6JWU VYQ CVQOU QH QZ[IGP UJCTG VYQ GNGEVTQPU GCEJ
VQ CVVCKP UVCDNG QEVGV EQPſIWTCVKQPU 6JKU NGCFU VQ VJG HQTOCVKQP QH C FQWDNG
bond between them. These two atoms of oxygen do not require any more
GNGEVTQPU DGECWUG VJG[ JCXG CNTGCF[ CVVCKPGF UVCDNG EQPſIWTCVKQPU #P[
of these two oxygen atoms can, however, donate a pair of electrons to the
third oxygen atom without sharing any of the electrons of the third oxygen
atom. As a result, a coordinate bond is formed between two oxygen atoms
CU UJQYP DGNQY