Page 85 - Chemistry ICSE Class X
P. 85
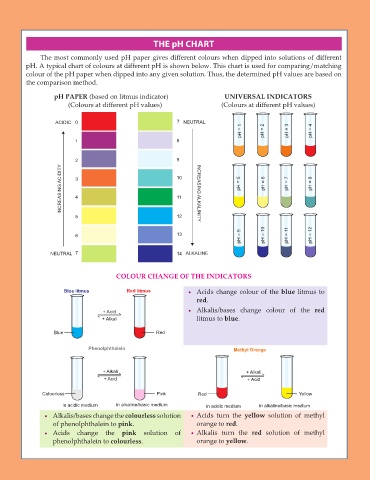
THE pH CHART
The most commonly used pH paper gives different colours when dipped into solutions of different
pH. A typical chart of colours at different pH is shown below. This chart is used for comparing/matching
colour of the pH paper when dipped into any given solution. Thus, the determined pH values are based on
the comparison method.
pH PAPER (based on litmus indicator) UNIVERSAL INDICATORS
(Colours at different pH values) (Colours at different pH values)
ACIDIC 0 7 NEUTRAL
pH = 1 pH = 2 pH = 3 pH = 4
1 8
2 9
INCREASING ACIDITY 3 4 10 INCREASING ALKALINITY pH = 5 pH = 6 pH = 7 pH = 8
11
5 12
pH = 9
6 13 pH = 10 pH = 11 pH = 12
NEUTRAL 7 14 ALKALINE
COLOUR CHANGE OF THE INDICATORS
Blue litmus Red litmus x Acids change colour of the blue litmus to
red.
+ Acid x Alkalis/bases change colour of the red
+ Alkali litmus to blue.
Blue Red
Phenolphthalein Methyl Orange
+ Alkali + Alkali
+ Acid + Acid
Colourless Pink Red Yellow
in acidic medium in alkaline/basic medium in acidic medium in alkaline/basic medium
x Alkalis/bases change the colourless solution x Acids turn the yellow solution of methyl
of phenolphthalein to pink. orange to red.
x Acids change the pink solution of x Alkalis turn the red solution of methyl
phenolphthalein to colourless. orange to yellow.