Page 145 - Chemistry ICSE Class IX
P. 145
The Periodic Table 133
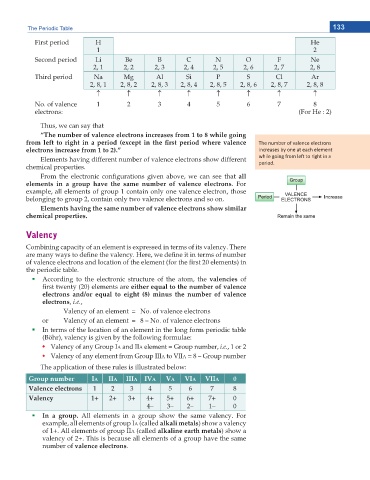
First period H He
1 2
Second period Li Be B C N O F Ne
2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8
Third period Na Mg Al Si P S Cl Ar
2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2, 8, 5 2, 8, 6 2, 8, 7 2, 8, 8
n n n n n n n n
No. of valence 1 2 3 4 5 6 7 8
electrons: (For He : 2)
Thus, we can say that
“The number of valence electrons increases from 1 to 8 while going
HTQO NGHV VQ TKIJV KP C RGTKQF GZEGRV KP VJG ſTUV RGTKQF YJGTG XCNGPEG dŚĞ ŶƵŵďĞƌ ŽĨ ǀĂůĞŶĐĞ ĞůĞĐƚƌŽŶƐ
electrons increase from 1 to 2).” ŝŶĐƌĞĂƐĞƐ ďLJ ŽŶĞ Ăƚ ĞĂĐŚ ĞůĞŵĞŶƚ
Elements having different number of valence electrons show different ǁŚŝůĞ ŐŽŝŶŐ ĨƌŽŵ ůĞŌ ƚŽ ƌŝŐŚƚ ŝŶ Ă
chemical properties. ƉĞƌŝŽĚ͘
From the electronic con gurations given above, we can see that all
elements in a group have the same number of valence electrons. For
example, all elements of group 1 contain only one valence electron, those
belonging to group 2, contain only two valence electrons and so on.
Elements having the same number of valence electrons show similar
chemical properties.
Valency
Combining capacity of an element is expressed in terms of its valency. There
are many ways to de ne the valency. Here, we de ne it in terms of number
of valence electrons and location of the element (for the rst 20 elements) in
the periodic table.
According to the electronic structure of the atom, the valencies of
rst twenty (20) elements are either equal to the number of valence
electrons and/or equal to eight (8) minus the number of valence
electrons, i.e.,
Valency of an element = No. of valence electrons
or Valency of an element = 8 – No. of valence electrons
In terms of the location of an element in the long form periodic table
(Böhr), valency is given by the following formulae:
Valency of any Group IA and IIA element = Group number, i.e., 1 or 2
Valency of any element from Group IIIA to VIIA = 8 – Group number
The application of these rules is illustrated below:
Group number IA IIA IIIA IVA VA VIA VIIA 0
Valence electrons 1 2 3 4 5 6 7 8
Valency 1+ 2+ 3+ 4+ 5+ 6+ 7+ 0
4– 3– 2– 1– 0
In a group. All elements in a group show the same valency. For
example, all elements of group IA (called alkali metals) show a valency
of 1+. All elements of group IIA (called alkaline earth metals) show a
valency of 2+. This is because all elements of a group have the same
number of valence electrons.