Page 146 - Chemistry ICSE Class IX
P. 146
134 ICSE Chemistry – 9
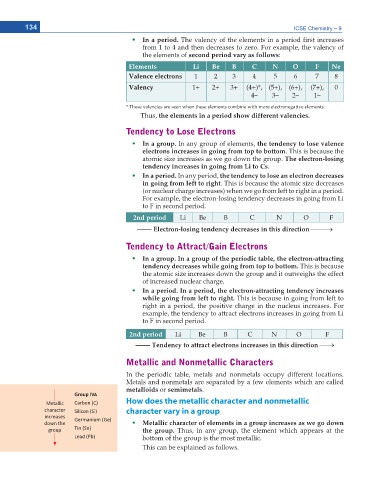
In a period. The valency of the elements in a period rst increases
from 1 to 4 and then decreases to zero. For example, the valency of
the elements of second period vary as follows:
Elements Li Be B C N O F Ne
Valence electrons 1 2 3 4 5 6 7 8
Valency 1+ 2+ 3+ (4+)*, (5+), (6+), (7+), 0
4– 3– 2– 1–
* These valencies are seen when these elements combine with more electronegative elements.
Thus, the elements in a period show different valencies.
Tendency to Lose Electrons
In a group. In any group of elements, the tendency to lose valence
electrons increases in going from top to bottom. This is because the
atomic size increases as we go down the group. The electron-losing
tendency increases in going from Li to Cs.
In a period. In any period, the tendency to lose an electron decreases
in going from left to right. This is because the atomic size decreases
(or nuclear charge increases) when we go from left to right in a period.
For example, the electron-losing tendency decreases in going from Li
to F in second period.
2nd period Li Be B C N O F
—— Electron-losing tendency decreases in this direction o
Tendency to Attract/Gain Electrons
In a group. In a group of the periodic table, the electron-attracting
tendency decreases while going from top to bottom. This is because
the atomic size increases down the group and it outweighs the effect
of increased nuclear charge.
In a period. In a period, the electron-attracting tendency increases
while going from left to right. This is because in going from left to
right in a period, the positive charge in the nucleus increases. For
example, the tendency to attract electrons increases in going from Li
to F in second period.
2nd period Li Be B C N O F
—— Tendency to attract electrons increases in this direction o
Metallic and Nonmetallic Characters
In the periodic table, metals and nonmetals occupy different locations.
Metals and nonmetals are separated by a few elements which are called
metalloids or semimetals.
Group IV
DĞƚĂůůŝĐ ĂƌďŽŶ ; Ϳ How does the metallic character and nonmetallic
ĐŚĂƌĂĐƚĞƌ ^ŝůŝĐŽŶ ;^ŝͿ character vary in a group
ŝŶĐƌĞĂƐĞƐ 'ĞƌŵĂŶŝƵŵ ;'ĞͿ
ĚŽǁŶ ƚŚĞ Metallic character of elements in a group increases as we go down
ŐƌŽƵƉ dŝŶ ;^ŶͿ the group. Thus, in any group, the element which appears at the
>ĞĂĚ ;WďͿ bottom of the group is the most metallic.
This can be explained as follows.