Page 60 - Chemistry ICSE Class IX
P. 60
48 ICSE Chemistry – 9
Table 2.2 Differences between combustion and respiration
Combustion Respiration
(Rapid combustion) (Slow combustion or spontaneous combustion)
1. Combustion is a fast process. 1. Respiration is a slow process.
2. Combustion starts only when the combustible 2. Respiration takes place at the body temperature
material is heated to its ignition temperature. (37°C). Therefore, no preheating is needed.
3. The liberation of heat during combustion is very 3. Heat is released so slowly that it is lost to the
rapid. It raises temperature of the burning material. surroundings and the temperature of the body
remains virtually unchanged.
4. Combustion is accompanied by ame and smoke. 4. No ame and smoke is seen during respiration (or
spontaneous combustion).
5. Combustion of any combustible material takes place 5. Respiration takes place inside the body of a human
outside the body of a human being or an animal. being or an animal.
How does respiration resemble combustion
Respiration is similar to combustion in certain respects. Some similarities
between the two are:
Respiration, like combustion, involves reaction of a combustible
material with oxygen.
The products of respiration and that of combustion are the same, viz,
CO , H O and heat energy.
2
2
How does respiration compare with combustion
A few points of comparison between respiration and combustion are given
in Table 2.3.
Table 2.3 Comparison between combustion and respiration
Property Combustion Respiration
1. Nature of reaction Oxidation Oxidation
2. Temperature High Body temperature (37°C)
3. Rate of process Fast Slow
4. Products Depend upon the reaction conditions, but Always only CO and H O
2
2
generally CO , H O, and heat and light
2
2
Change in Mass on Burning
When a substance is burnt, the substance combines with oxygen to give
one or more products. If the experiment is carried out in such a way that
all the products are collected (without any loss) and weighed precisely,
then the total mass of all the products will be greater than the mass of the
substance burnt.
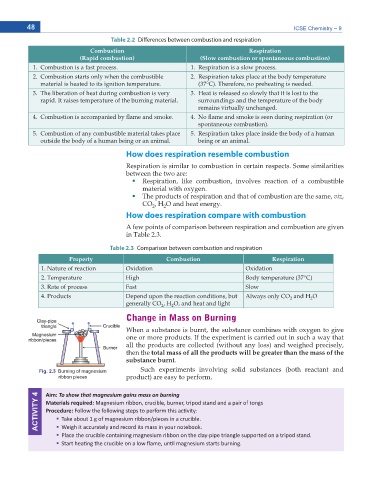
Fig. 2.3 Burning of magnesium Such experiments involving solid substances (both reactant and
ribbon pieces product) are easy to perform.
ACTIVITY 4 Aim: dŽ ƐŚŽǁ ƚŚĂƚ ŵĂŐŶĞƐŝƵŵ ŐĂŝŶƐ ŵĂƐƐ ŽŶ ďƵƌŶŝŶŐ
Materials required: DĂŐŶĞƐŝƵŵ ƌŝďďŽŶ͕ ĐƌƵĐŝďůĞ͕ ďƵƌŶĞƌ͕ ƚƌŝƉŽĚ ƐƚĂŶĚ ĂŶĚ Ă ƉĂŝƌ ŽĨ ƚŽŶŐƐ
Procedure: &ŽůůŽǁ ƚŚĞ ĨŽůůŽǁŝŶŐ ƐƚĞƉƐ ƚŽ ƉĞƌĨŽƌŵ ƚŚŝƐ ĂĐƟǀŝƚLJ͗
dĂŬĞ ĂďŽƵƚ ϭ Ő ŽĨ ŵĂŐŶĞƐŝƵŵ ƌŝďďŽŶͬƉŝĞĐĞƐ ŝŶ Ă ĐƌƵĐŝďůĞ͘
WůĂĐĞ ƚŚĞ ĐƌƵĐŝďůĞ ĐŽŶƚĂŝŶŝŶŐ ŵĂŐŶĞƐŝƵŵ ƌŝďďŽŶ ŽŶ ƚŚĞ ĐůĂLJͲƉŝƉĞ ƚƌŝĂŶŐůĞ ƐƵƉƉŽƌƚĞĚ ŽŶ Ă ƚƌŝƉŽĚ ƐƚĂŶĚ͘
tĞŝŐŚ ŝƚ ĂĐĐƵƌĂƚĞůLJ ĂŶĚ ƌĞĐŽƌĚ ŝƚƐ ŵĂƐƐ ŝŶ LJŽƵƌ ŶŽƚĞŬ͘
^ƚĂƌƚ ŚĞĂƟŶŐ ƚŚĞ ĐƌƵĐŝďůĞ ŽŶ Ă ůŽǁ ŇĂŵĞ͕ ƵŶƟů ŵĂŐŶĞƐŝƵŵ ƐƚĂƌƚƐ ďƵƌŶŝŶŐ͘