Page 98 - Chemistry ICSE Class IX
P. 98
86 ICSE Chemistry – 9
This uorescence of the walls is due to the bombardment of the glass
by some rays emitted from the cathode. Therefore, these rays are called
cathode rays.
What are the properties of cathode rays
Cathode rays show the following properties:
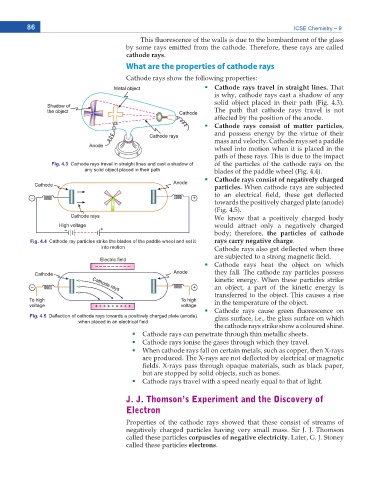
Cathode rays travel in straight lines. That
is why, cathode rays cast a shadow of any
solid object placed in their path (Fig. 4.3).
The path that cathode rays travel is not
affected by the position of the anode.
Cathode rays consist of matter particles,
and possess energy by the virtue of their
mass and velocity. Cathode rays set a paddle
wheel into motion when it is placed in the
path of these rays. This is due to the impact
Fig. 4.3 Cathode rays travel in straight lines and cast a shadow of of the particles of the cathode rays on the
any solid object placed in their path blades of the paddle wheel (Fig. 4.4).
Cathode rays consist of negatively charged
particles. When cathode rays are subjected
to an electrical eld, these get de ected
towards the positively charged plate (anode)
(Fig. 4.5).
We know that a positively charged body
would attract only a negatively charged
body; therefore, the particles of cathode
Fig. 4.4 Cathode ray particles strike the blades of the paddle wheel and set it rays carry negative charge.
into motion Cathode rays also get de ected when these
are subjected to a strong magnetic eld.
Cathode rays heat the object on which
they fall. The cathode ray particles possess
kinetic energy. When these particles strike
an object, a part of the kinetic energy is
transferred to the object. This causes a rise
in the temperature of the object.
Cathode rays cause green uorescence on
Fig. 4.5 DeÀection of cathode rays towards a positively charged plate (anode) glass surface, i.e., the glass surface on which
when placed in an electrical ¿eld
the cathode rays strike show a coloured shine.
Cathode rays can penetrate through thin metallic sheets.
Cathode rays ionise the gases through which they travel.
When cathode rays fall on certain metals, such as copper, then X-rays
are produced. The X-rays are not de ected by electrical or magnetic
elds. X-rays pass through opaque materials, such as black paper,
but are stopped by solid objects, such as bones.
Cathode rays travel with a speed nearly equal to that of light.
J. J. Thomson’s Experiment and the Discovery of
Electron
Properties of the cathode rays showed that these consist of streams of
negatively charged particles having very small mass. Sir J. J. Thomson
called these particles corpuscles of negative electricity. Later, G. J. Stoney
called these particles electrons.