Page 126 - Chemistry ICSE Class X
P. 126
112 ICSE Chemistry – 10
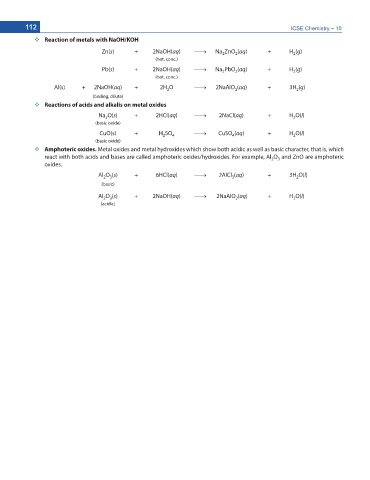
Reaction of metals with NaOH/KOH
Zn(s) + 2NaOH(aq) o Na ZnO (aq) + H (g)
2 2 2
(hot, conc.)
Pb(s) + 2NaOH(aq) o Na PbO (aq) + H (g)
2
2
2
(hot, conc.)
Al(s) + 2NaOH(aq) + 2H O o 2NaAlO (aq) + 3H (g)
2 2 2
(boiling, dilute)
Reactions of acids and alkalis on metal oxides
Na O(s) + 2HCl(aq) o 2NaCl(aq) + H O(l)
2 2
(basic oxide)
CuO(s) + H SO o CuSO (aq) + H O(l)
2
4
4
2
(basic oxide)
Amphoteric oxides. Metal oxides and metal hydroxides which show both acidic as well as basic character, that is, which
react with both acids and bases are called amphoteric oxides/hydroxides. For example, Al O and ZnO are amphoteric
2 3
oxides.
Al O (s) + 6HCl(aq) o 2AlCl (aq) + 3H O(l)
3
2
2 3
(basic)
Al O (s) + 2NaOH(aq) o 2NaAlO (aq) + H O(l)
2
2 3
2
(acidic)