Page 15 - Chemistry ICSE Class X
P. 15
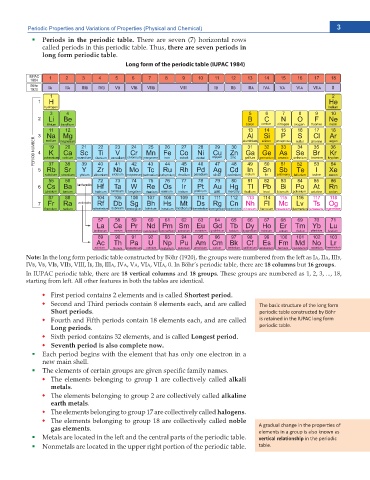
Periodic Properties and Variations of Properties (Physical and Chemical) 3
Periods in the periodic table. There are seven (7) horizontal rows
ECNNGF RGTKQFU KP VJKU RGTKQFKE VCDNG 6JWU there are seven periods in
long form periodic table
Long form of the periodic table (IUPAC 1984)
Note: In the long form periodic table constructed by Böhr (1920), the groups were numbered from the left as IA, IIA, IIIB,
IVB, VB, VIB, VIIB, VIII, IB, IIB, IIIA, IVA, VA, VIA, VIIA +P $ÑJTŏU RGTKQFKE VCDNG VJGTG CTG 18 columns but 16 groups
In IUPAC periodic table, there are 18 vertical columns and 18 groups 6JGUG ITQWRU CTG PWODGTGF CU
UVCTVKPI HTQO NGHV #NN QVJGT HGCVWTGU KP DQVJ VJG VCDNGU CTG KFGPVKECN
First period contains 2 elements and is called Shortest period
Second and Third periods contain 8 elements each, and are called dŚĞ ďĂƐŝĐ ƐƚƌƵĐƚƵƌĞ ŽĨ ƚŚĞ ůŽŶŐ ĨŽƌŵ
Short periods ƉĞƌŝŽĚŝĐ ƚĂďůĞ ĐŽŶƐƚƌƵĐƚĞĚ ďLJ ƂŚƌ
Fourth and Fifth periods contain 18 elements each, and are called ŝƐ ƌĞƚĂŝŶĞĚ ŝŶ ƚŚĞ /hW ůŽŶŐ ĨŽƌŵ
Long periods ƉĞƌŝŽĚŝĐ ƚĂďůĞ͘
Sixth period contains 32 elements, and is called Longest period
Seventh period is also complete now.
Each period begins with the element that has only one electron in a
PGY OCKP UJGNN
6JG GNGOGPVU QH EGTVCKP ITQWRU CTG IKXGP URGEKſE HCOKN[ PCOGU
The elements belonging to group 1 are collectively called alkali
metals
The elements belonging to group 2 are collectively called alkaline
earth metals
The elements belonging to group 17 are collectively called halogens
The elements belonging to group 18 are collectively called noble
gas elements ŐƌĂĚƵĂů ĐŚĂŶŐĞ ŝŶ ƚŚĞ ƉƌŽƉĞƌƟĞƐ ŽĨ
ĞůĞŵĞŶƚƐ ŝŶ Ă ŐƌŽƵƉ ŝƐ ĂůƐŽ ŬŶŽǁŶ ĂƐ
/GVCNU CTG NQECVGF KP VJG NGHV CPF VJG EGPVTCN RCTVU QH VJG RGTKQFKE VCDNG ǀĞƌƟĐĂů ƌĞůĂƟŽŶƐŚŝƉ ŝŶ ƚŚĞ ƉĞƌŝŽĚŝĐ
0QPOGVCNU CTG NQECVGF KP VJG WRRGT TKIJV RQTVKQP QH VJG RGTKQFKE VCDNG ƚĂďůĞ͘