Page 168 - Chemistry ICSE Class X
P. 168
154 ICSE Chemistry – 10
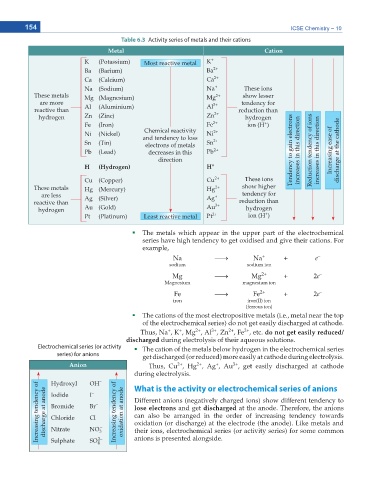
Table 6.3 Activity series of metals and their cations
Metal Cation
K (Potassium) Most reactive metal K +
Ba (Barium) Ba 2+
Ca (Calcium) Ca 2+
Na (Sodium) Na + These ions
These metals Mg (Magnesium) Mg 2+ show lesser
are more 3+ tendency for
reactive than Al (Aluminium) Al reduction than
hydrogen Zn (Zinc) Zn 2+ hydrogen
+
Fe (Iron) Fe 2+ ion (H )
Chemical reactivity
Ni (Nickel) and tendency to loss Ni 2+
Sn (Tin) electrons of metals Sn 2+
Pb (Lead) decreases in this Pb 2+ Tendency to gain electrons increases in this direction Reduction tendency of ions increases in this direction Increasing ease of discharge at the cathode
direction
H (Hydrogen) H +
Cu (Copper) Cu 2+ These ions
These metals Hg (Mercury) Hg 2+ show higher
are less Ag (Silver) Ag + tendency for
reactive than 3+ reduction than
hydrogen Au (Gold) Au hydrogen
+
Pt (Platinum) Least reactive metal Pt 2+ ion (H )
The metals which appear in the upper part of the electrochemical
series have high tendency to get oxidised and give their cations. For
example,
Na o Na + + e –
sodium sodium ion
Mg o Mg 2+ + 2e –
Magnesium magnesium ion
Fe o Fe 2+ + 2e –
iron iron(II) ion
(ferrous ion)
The cations of the most electropositive metals (i.e., metal near the top
of the electrochemical series) do not get easily discharged at cathode.
2+
+
3+
+
2+
2+
Thus, Na , K , Mg , Al , Zn , Fe , etc. do not get easily reduced/
discharged during electrolysis of their aqueous solutions.
Electrochemical series (or activity The cation of the metals below hydrogen in the electrochemical series
series) for anions get discharged (or reduced) more easily at cathode during electrolysis.
2+
+
2+
3+
Anion Thus, Cu , Hg , Ag , Au , get easily discharged at cathode
during electrolysis.
–
OH
Hydroxyl
Increasing tendency of discharge at anode Iodide I Br – – 2– 3 – Increasing tendency of oxidation at anode Different anions (negatively charged ions) show different tendency to
What is the activity or electrochemical series of anions
–
Bromide
lose electrons and get discharged at the anode. Therefore, the anions
can also be arranged in the order of increasing tendency towards
Cl
Chloride
oxidation (or discharge) at the electrode (the anode). Like metals and
Nitrate
NO
their ions, electrochemical series (or activity series) for some common
anions is presented alongside.
SO
Sulphate
4