Page 228 - Chemistry ICSE Class IX
P. 228
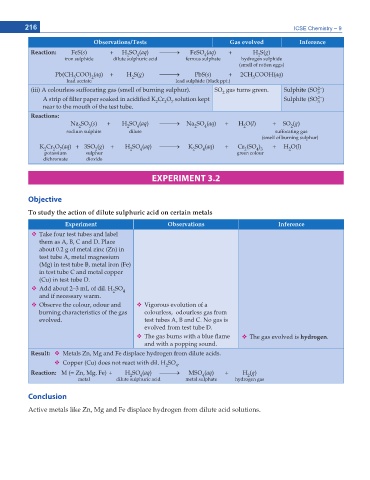
216 ICSE Chemistry – 9
Observations/Tests Gas evolved Inference
Reaction: FeS(s) + H SO (aq) o FeSO (aq) + H S(g)
2
4
2
4
iron sulphide dilute sulphuric acid ferrous sulphate hydrogen sulphide
(smell of rotten eggs)
Pb(CH COO) (aq) + H S(g) o PbS(s) + 2CH COOH(aq)
3
2
2
3
lead acetate lead sulphide (black ppt.)
2–
(iii) A colourless suffocating gas (smell of burning sulphur). SO gas turns green. Sulphite (SO )
2 3
A strip of lter paper soaked in acidi ed K Cr O solution kept Sulphite (SO )
2–
3
2
7
2
near to the mouth of the test tube.
Reactions:
Na SO (s) + H SO (aq) o Na SO (aq) + H O(l) + SO (g)
2
2
3
2
4
2
4
2
sodium sulphite dilute suffocating gas
(smell of burning sulphur)
K Cr O (aq) + 3SO (g) + H SO (aq) o K SO (aq) + Cr (SO ) + H O(l)
2
2
2
4
2
4 3
4
7
2
2
2
potassium sulphur green colour
dichromate dioxide
EXPERIMENT 3.2
Objective
6Q UVWF[ VJG CEVKQP QH FKNWVG UWNRJWTKE CEKF QP EGTVCKP OGVCNU
'ZRGTKOGPV Observations Inference
Take four test tubes and label
them as A, B, C and D. Place
about 0.2 g of metal zinc (Zn) in
test tube A, metal magnesium
(Mg) in test tube B, metal iron (Fe)
in test tube C and metal copper
(Cu) in test tube D.
Add about 2–3 mL of dil. H SO
4
2
and if necessary warm.
Observe the colour, odour and Vigorous evolution of a
burning characteristics of the gas colourless, odourless gas from
evolved. test tubes A, B and C. No gas is
evolved from test tube D.
The gas burns with a blue ame The gas evolved is hydrogen.
and with a popping sound.
Result: Metals Zn, Mg and Fe displace hydrogen from dilute acids.
Copper (Cu) does not react with dil. H SO .
4
2
Reaction: M (= Zn, Mg, Fe) + H SO (aq) o MSO (aq) + H (g)
2
4
4
2
metal dilute sulphuric acid metal sulphate hydrogen gas
Conclusion
Active metals like Zn, Mg and Fe displace hydrogen from dilute acid solutions.