Page 227 - Chemistry ICSE Class IX
P. 227
3 Action of Dilute Sulphuric
Acid on a Substance
BASIC CONCEPTS
Dilute sulphuric acid (or hydrochloric acid) decomposes certain salts liberating characteristic gases. Active
metals, such as zinc or magnesium, also react with dilute sulphuric acid (or hydrochloric acid) liberating
hydrogen gas. These evolved gases can be identi ed by simple tests. These tests are described below.
Anions indicated by dilute sulphuric acid
The salts of certain anions are decomposed by dil. H SO . Such decompositions are accompanied by the
2
4
evolution of certain gases. These anions (acid radicals) can be identi ed by performing tests on the evolved
gases. The anions which can be detected by this test are
CO 2– S 2– SO 2– NO – CH COO –
3 3 2 3
carbonate sulphide sulphite nitrite acetate
2–
2–
2–
But only the anions CO , S and SO are to be considered at this level (as per syllabus).
3 3
Active metals, such as zinc, magnesium and iron, react with dilute hydrochloric acid [HCl(aq)] and dilute
sulphuric acid [H SO (aq)] liberating hydrogen gas.
2
4
EXPERIMENT 3.1
Objective
To study the action of dilute hydrochloric acid or sulphuric acid on certain salts and identify the anion
RTGUGPV KP VJGO
Procedure
Take about 0.2 g of the salt in a test tube, and add about 1 mL of dilute H SO (or dilute HCl). Note, if a
4
2
reaction takes place in cold. If not, warm (do not heat strongly), and observe for the occurrence of any reaction.
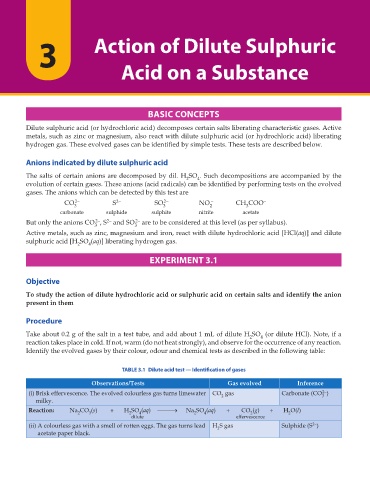
Identify the evolved gases by their colour, odour and chemical tests as described in the following table:
TABLE 3.1 Dilute acid test — Identification of gases
Observations/Tests Gas evolved Inference
2–
(i) Brisk effervescence. The evolved colourless gas turns limewater CO gas Carbonate (CO )
2
3
milky.
Reaction: Na CO (s) + H SO (aq) o Na SO (aq) + CO (g) + H O(l)
2 3 2 4 2 4 2 2
dilute effervescence
2–
(ii) A colourless gas with a smell of rotten eggs. The gas turns lead H S gas Sulphide (S )
2
acetate paper black.