Page 225 - Chemistry ICSE Class IX
P. 225
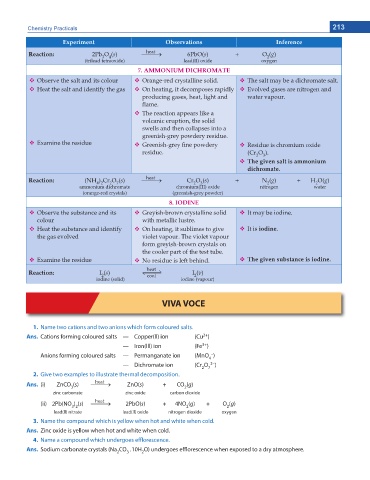
Chemistry Practicals 213
'ZRGTKOGPV Observations Inference
heat
Reaction: 2Pb O (s) o 6PbO(s) + O (g)
2
4
3
(trilead tetraoxide) lead(II) oxide oxygen
7. AMMONIUM DICHROMATE
Observe the salt and its colour Orange-red crystalline solid. The salt may be a dichromate salt.
Heat the salt and identify the gas On heating, it decomposes rapidly Evolved gases are nitrogen and
producing gases, heat, light and water vapour.
ame.
The reaction appears like a
volcanic eruption, the solid
swells and then collapses into a
greenish-grey powdery residue.
Examine the residue Greenish-grey ne powdery Residue is chromium oxide
residue. (Cr O ).
2
3
6JG IKXGP UCNV KU COOQPKWO
FKEJTQOCVG
heat
Reaction: (NH ) Cr O (s) o Cr O (s) + N (g) + H O(g)
2
2
4 2
7
3
2
2
ammonium dichromate chromium(III) oxide nitrogen water
(orange-red crystals) (greenish-grey powder)
8. IODINE
Observe the substance and its Greyish-brown crystalline solid It may be iodine.
colour with metallic lustre.
Heat the substance and identify On heating, it sublimes to give It is iodine.
the gas evolved violet vapour. The violet vapour
form greyish-brown crystals on
the cooler part of the test tube.
Examine the residue No residue is left behind. The given substance is iodine.
heat
Reaction: I (s) cool I (v)
2
2
iodine (solid) iodine (vapour)
VIVA VOCE
1. Name two cations and two anions which form coloured salts.
2+
Ans. Cations forming coloured salts — Copper(ll) ion (Cu )
3+
— Iron(lll) ion (Fe )
–
Anions forming coloured salts — Permanganate ion (MnO )
4
— Dichromate ion (Cr O 2– )
2 7
2. Give two examples to illustrate thermal decomposition.
heat
Ans. (i) ZnCO (s) o ZnO(s) + CO (g)
3 2
zinc carbonate zinc oxide carbon dioxide
heat
(ii) 2Pb(NO ) (s) o 2PbO(s) + 4NO (g) + O (g)
3 2 2 2
lead(ll) nitrate lead(ll) oxide nitrogen dioxide oxygen
3. Name the compound which is yellow when hot and white when cold.
Ans. Zinc oxide is yellow when hot and white when cold.
4. Name a compound which undergoes efflorescence.
Ans. Sodium carbonate crystals (Na CO .10H O) undergoes efflorescence when exposed to a dry atmosphere.
2 3 2