Page 221 - Chemistry ICSE Class IX
P. 221
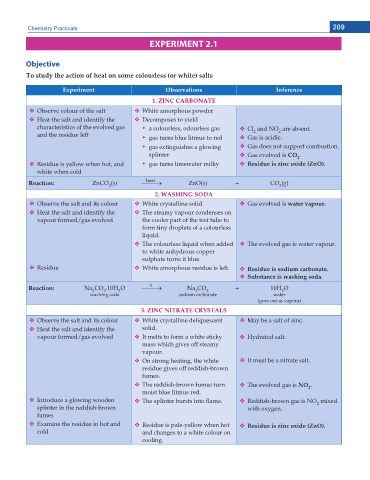
Chemistry Practicals 209
EXPERIMENT 2.1
Objective
To study the action of heat on some colourless (or white) salts
'ZRGTKOGPV Observations Inference
1. ZINC CARBONATE
Observe colour of the salt White amorphous powder
Heat the salt and identify the Decomposes to yield
characteristics of the evolved gas y a colourless, odourless gas Cl and NO are absent.
2
2
and the residue left y gas turns blue litmus to red Gas is acidic.
y gas extinguishes a glowing Gas does not support combustion.
splinter Gas evolved is CO .
2
Residue is yellow when hot, and y gas turns limewater milky 4GUKFWG KU \KPE QZKFG <P1
white when cold
heat
Reaction: ZnCO (s) o ZnO(s) + CO (g)
3 2
2. WASHING SODA
Observe the salt and its colour White crystalline solid. Gas evolved is water vapour.
Heat the salt and identify the The steamy vapour condenses on
vapour formed/gas evolved the cooler part of the test tube to
form tiny droplets of a colourless
liquid.
The colourless liquid when added The evolved gas is water vapour.
to white anhydrous copper
sulphate turns it blue.
Residue White amorphous residue is left. 4GUKFWG KU UQFKWO ECTDQPCVG
Substance is washing soda.
'
.
Reaction: Na CO 10H O o Na CO 3 + 10H O
3
2
2
2
2
washing soda sodium carbonate water
(goes out as vapour)
3. ZINC NITRATE CRYSTALS
Observe the salt and its colour White crystalline deliquescent May be a salt of zinc.
Heat the salt and identify the solid.
vapour formed/gas evolved It melts to form a white sticky Hydrated salt.
mass which gives off steamy
vapour.
On strong heating, the white It must be a nitrate salt.
residue gives off reddish-brown
fumes.
The reddish-brown fumes turn The evolved gas is NO .
2
moist blue litmus red.
Introduce a glowing wooden The splinter bursts into ame. Reddish-brown gas is NO mixed
splinter in the reddish-brown with oxygen. 2
fumes
Examine the residue in hot and Residue is pale-yellow when hot 4GUKFWG KU \KPE QZKFG <P1
cold and changes to a white colour on
cooling.