Page 217 - Chemistry ICSE Class IX
P. 217
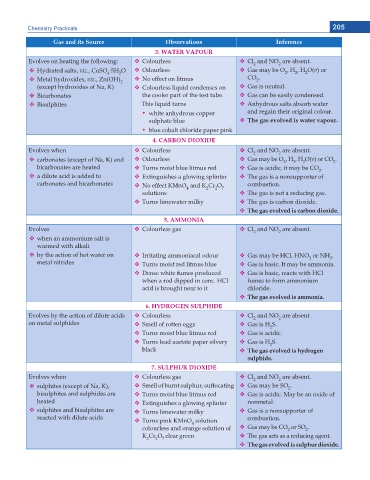
Chemistry Practicals 205
Gas and its Source Observations Inference
3. WATER VAPOUR
Evolves on heating the following: Colourless Cl and NO are absent.
2
2
.
Hydrated salts, viz., CuSO 5H O Odourless Gas may be O , H , H O(v) or
2
2
2
2
4
Metal hydroxides, viz., Zn(OH) No effect on litmus CO .
2
2
(except hydroxides of Na, K) Colourless liquid condenses on Gas is neutral.
Bicarbonates the cooler part of the test tube. Gas can be easily condensed.
Bisulphites This liquid turns Anhydrous salts absorb water
y white anhydrous copper and regain their original colour.
sulphate blue The gas evolved is water vapour.
y blue cobalt chloride paper pink
4. CARBON DIOXIDE
Evolves when Colourless Cl and NO are absent.
2 2
carbonates (except of Na, K) and Odourless Gas may be O , H , H O(v) or CO .
2
2
2
2
bicarbonates are heated Turns moist blue litmus red Gas is acidic, it may be CO .
2
a dilute acid is added to Extinguishes a glowing splinter The gas is a nonsupporter of
carbonates and bicarbonates No effect KMnO and K Cr O combustion.
solutions 4 2 2 7 The gas is not a reducing gas.
Turns limewater milky The gas is carbon dioxide.
The gas evolved is carbon dioxide.
5. AMMONIA
Evolves Colourless gas Cl and NO are absent.
2 2
when an ammonium salt is
warmed with alkali
by the action of hot water on Irritating ammoniacal odour Gas may be HCl, HNO or NH .
3
3
metal nitrides Turns moist red litmus blue Gas is basic. It may be ammonia.
Dense white fumes produced Gas is basic, reacts with HCl
when a rod dipped in conc. HCl fumes to form ammonium
acid is brought near to it chloride.
6JG ICU GXQNXGF KU COOQPKC
6. HYDROGEN SULPHIDE
Evolves by the action of dilute acids Colourless Cl and NO are absent
2
2
on metal sulphides Smell of rotten eggs Gas is H S.
2
Turns moist blue litmus red Gas is acidic.
Turns lead acetate paper silvery Gas is H S.
2
black The gas evolved is hydrogen
sulphide.
7. SULPHUR DIOXIDE
Evolves when Colourless gas Cl and NO are absent.
2
2
sulphites (except of Na, K), Smell of burnt sulphur, suffocating Gas may be SO .
2
bisulphites and sulphides are Turns moist blue litmus red Gas is acidic. May be an oxide of
heated Extinguishes a glowing splinter nonmetal.
sulphites and bisulphites are Turns limewater milky Gas is a nonsupporter of
reacted with dilute acids combustion.
Turns pink KMnO solution
4
colourless and orange solution of Gas may be CO or SO .
2
2
K Cr O clear green The gas acts as a reducing agent.
2 2 7
The gas evolved is sulphur dioxide.