Page 220 - Chemistry ICSE Class IX
P. 220
2 Action of Heat on Some
Substances or Salts
BASIC CONCEPTS
Whenever a substance/salt is heated, it undergoes some characteristic changes. These changes may include
one or more of the following:
y Giving off (or losing) water of crystallisation.
y Change in colour
y Decompose to give a gas/vapour. The vapour evolved may be colourless or coloured.
y Sublimation of the substance.
From these observations, one can identify the given salt.
y Certain salts are white, whereas some others are coloured.
y The colour of the salt is primarily due to the cation present in it.
y Certain oxyanions also impart colour to the salt.
Some typical ions are given below.
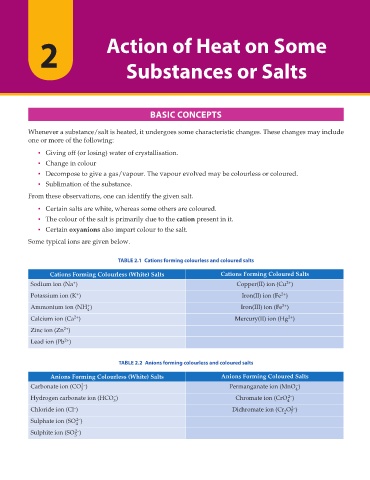
TABLE 2.1 Cations forming colourless and coloured salts
%CVKQPU (QTOKPI %QNQWTNGUU 9JKVG 5CNVU %CVKQPU (QTOKPI %QNQWTGF 5CNVU
2+
+
Sodium ion (Na ) Copper(II) ion (Cu )
2+
+
Potassium ion (K ) Iron(II) ion (Fe )
Ammonium ion (NH ) Iron(III) ion (Fe )
+
3+
4
2+
2+
Calcium ion (Ca ) Mercury(II) ion (Hg )
2+
Zinc ion (Zn )
Lead ion (Pb )
2+
TABLE 2.2 Anions forming colourless and coloured salts
#PKQPU (QTOKPI %QNQWTNGUU 9JKVG 5CNVU #PKQPU (QTOKPI %QNQWTGF 5CNVU
–
2–
Carbonate ion (CO ) Permanganate ion (MnO )
4
3
Hydrogen carbonate ion (HCO ) Chromate ion (CrO )
2–
–
4
3
2–
Chloride ion (Cl ) Dichromate ion (Cr O )
–
7
2
Sulphate ion (SO )
2–
4
2–
Sulphite ion (SO )
3