Page 223 - Chemistry ICSE Class IX
P. 223
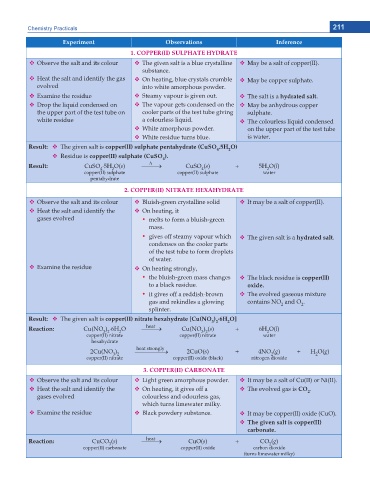
Chemistry Practicals 211
'ZRGTKOGPV Observations Inference
%122'4 ++ 57.2*#6' *;&4#6'
Observe the salt and its colour The given salt is a blue crystalline May be a salt of copper(II).
substance.
Heat the salt and identify the gas On heating, blue crystals crumble May be copper sulphate.
evolved into white amorphous powder.
Examine the residue Steamy vapour is given out. The salt is a hydrated salt.
Drop the liquid condensed on The vapour gets condensed on the May be anhydrous copper
the upper part of the test tube on cooler parts of the test tube giving sulphate.
white residue a colourless liquid. The colourless liquid condensed
White amorphous powder. on the upper part of the test tube
White residue turns blue. is water.
Result: The given salt is EQRRGT ++ UWNRJCVG RGPVCJ[FTCVG %W51 .5H 1
4 2
Residue is EQRRGT ++ UWNRJCVG %W51
4
'
.
Result: CuSO 5H O(s) o CuSO (s) + 5H O(l)
2
4
4
2
copper(II) sulphate copper(II) sulphate water
pentahydrate
%122'4 ++ 0+64#6' *':#*;&4#6'
Observe the salt and its colour Bluish-green crystalline solid It may be a salt of copper(II).
Heat the salt and identify the On heating, it
gases evolved y melts to form a bluish-green
mass.
y gives off steamy vapour which The given salt is a hydrated salt.
condenses on the cooler parts
of the test tube to form droplets
of water.
Examine the residue On heating strongly,
y the bluish-green mass changes The black residue is EQRRGT ++
to a black residue. oxide.
y it gives off a reddish-brown The evolved gaseous mixture
gas and rekindles a glowing contains NO and O .
2
2
splinter.
.
Result: The given salt is EQRRGT ++ PKVTCVG JGZCJ[FTCVG =%W 01 6H O]
3 2
2
heat
.
Reaction: Cu(NO ) 6H O o Cu(NO ) (s) + 6H O(l)
3 2
2
2
3 2
copper(II) nitrate copper(II) nitrate water
hexahydrate
heat strongly
2Cu(NO ) o 2CuO(s) + 4NO (g) + H O(g)
2
2
3 2
copper(II) nitrate copper(II) oxide (black) nitrogen dioxide
%122'4 ++ %#4$10#6'
Observe the salt and its colour Light green amorphous powder. It may be a salt of Cu(II) or Ni(II).
Heat the salt and identify the On heating, it gives off a The evolved gas is CO .
2
gases evolved colourless and odourless gas,
which turns limewater milky.
Examine the residue Black powdery substance. It may be copper(II) oxide (CuO).
6JG IKXGP UCNV KU EQRRGT ++
carbonate.
heat
Reaction: CuCO (s) o CuO(s) + CO (g)
2
3
copper(II) carbonate copper(II) oxide carbon dioxide
(turns limewater milky)