Page 24 - Chemistry ICSE Class IX
P. 24
12 ICSE Chemistry – 9
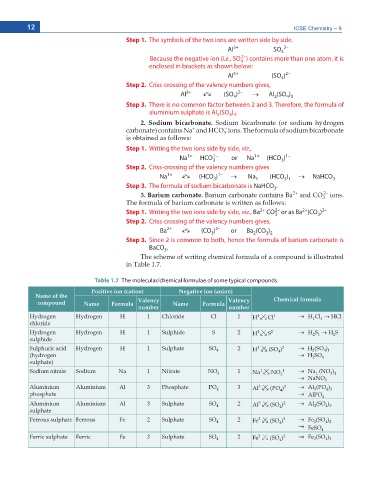
Step 1. The symbols of the two ions are written side by side.
Al 3+ SO 4 2–
2–
Because the negative ion (i.e., SO ) contains more than one atom, it is
4
enclosed in brackets as shown below:
Al 3+ (SO ) 2–
4
Step 2. Criss-crossing of the valency numbers gives,
3+
2–
Al o o (SO ) o Al (SO )
4
4 3
2
Step 3. There is no common factor between 2 and 3. Therefore, the formula of
aluminium sulphate is Al (SO ) .
2
4 3
2. Sodium bicarbonate. Sodium bicarbonate (or sodium hydrogen
–
+
carbonate) contains Na and HCO ions. The formula of sodium bicarbonate
3
is obtained as follows:
Step 1. Writing the two ions side by side, viz.,
1+
1+
Na HCO 3 1– or Na (HCO ) 1–
3
Step 2. Criss-crossing of the valency numbers gives
1+
1–
Na o o (HCO ) o Na (HCO ) o NaHCO
3
3 1
1
3
Step 3. The formula of sodium bicarbonate is NaHCO .
3
2+
2–
3. Barium carbonate. Barium carbonate contains Ba and CO ions.
3
The formula of barium carbonate is written as follows:
2+
2–
2+
Step 1. Writing the two ions side by side, viz., Ba CO or as Ba (CO ) 2–
3 3
Step 2. Criss-crossing of the valency numbers gives,
2+
2–
Ba o o (CO ) or Ba (CO )
2
3
3 2
Step 3. Since 2 is common to both, hence the formula of barium carbonate is
BaCO .
3
The scheme of writing chemical formula of a compound is illustrated
in Table 1.7.
Table 1.7 The molecular/chemical formulae of some typical compounds
Positive ion (cation) Negative ion (anion)
Name of the Chemical formula
compound Name Formula Valency Name Formula Valency
number number
Hydrogen Hydrogen H 1 Chloride Cl 1 H 02 Cl 1 o H Cl o HCl
1
1
1
chloride
Hydrogen Hydrogen H 1 Sulphide S 2 H 02 S o H S o H S
2
1
2
2 1
sulphide
1
Sulphuric acid Hydrogen H 1 Sulphate SO 4 2 H 02 (SO ) 2 o H (SO )
4 1
2
(hydrogen 4 o H SO 4
2
sulphate)
Sodium nitrate Sodium Na 1 Nitrate NO 3 1 Na 02 NO 3 1 o Na (NO )
1
1
3 1
o NaNO 3
Aluminium Aluminium Al 3 Phosphate PO 4 3 Al 02 (PO ) 3 o Al (PO )
3
3
4 3
phosphate 4 o AlPO
4
Aluminium Aluminium Al 3 Sulphate SO 4 2 Al 02 (SO ) 2 o Al (SO )
3
4 3
2
sulphate 4
Ferrous sulphate Ferrous Fe 2 Sulphate SO 4 2 Fe 02 (SO ) 2 o Fe (SO )
2
4 2
2
4
o FeSO 4
3
Ferric sulphate Ferric Fe 3 Sulphate SO 4 2 Fe 02 (SO ) 2 o Fe (SO )
2
4 3
4