Page 25 - Chemistry ICSE Class IX
P. 25
The Language of Chemistry 13
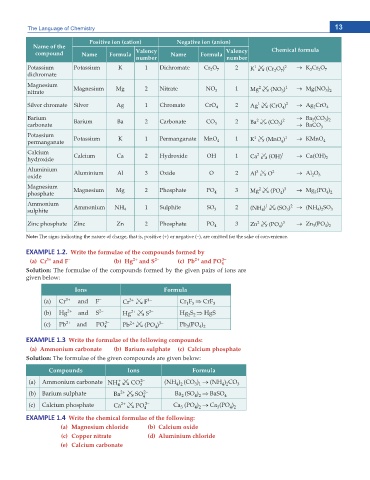
Positive ion (cation) Negative ion (anion)
Name of the Chemical formula
compound Name Formula Valency Name Formula Valency
number number
1
Potassium Potassium K 1 Dichromate Cr O 7 2 K 02 (Cr O ) 2 o K Cr O 7
2
2
2
dichromate 2 7
Magnesium Magnesium Mg 2 Nitrate NO 1 2 1 o Mg(NO )
nitrate 3 Mg 02 (NO ) 3 2
3
Silver chromate Silver Ag 1 Chromate CrO 4 2 Ag 02 (CrO ) 2 o Ag CrO 4
1
2
4
Barium 2 2 o Ba (CO )
2
3 2
carbonate Barium Ba 2 Carbonate CO 3 2 Ba 02 (CO ) o BaCO 3
3
Potassium Potassium K 1 Permanganate MnO 1 1 1 o KMnO
4
permanganate 4 K 02 (MnO ) 4
Calcium Calcium Ca 2 Hydroxide OH 1 2 1 o Ca(OH)
hydroxide Ca 02 (OH) 2
Aluminium Aluminium Al 3 Oxide O 2 3 2 o Al O
oxide Al 02 O 2 3
Magnesium 2 3
phosphate Magnesium Mg 2 Phosphate PO 4 3 Mg 02 (PO ) o Mg (PO )
3
4 2
4
Ammonium Ammonium NH 1 Sulphite SO 2 1 2 o (NH ) SO
4
3
sulphite 4 3 (NH ) 02 (SO ) 4 2 3
Zinc phosphate Zinc Zn 2 Phosphate PO 4 3 Zn 02 (PO ) 3 o Zn (PO )
2
3
4 2
4
Note: The signs indicating the nature of charge, that is, positive (+) or negative (–), are omitted for the sake of convenience.
EXAMPLE 1.2. Write the formulae of the compounds formed by
2+
2+
3+
(a) Cr and F – (b) Hg and S 2– (c) Pb and PO 3–
4
Solution: The formulae of the compounds formed by the given pairs of ions are
given below:
Ions Formula
3+
3+
(a) Cr and F – Cr 02 F 1– Cr F CrF 3
1 3
2+
(b) Hg and S 2– Hg 02 S 2– Hg S HgS
2+
2 2
2+
2+
(c) Pb and PO 4 3– Pb 02 (PO ) 3– Pb (PO )
3
4 2
4
EXAMPLE 1.3 Write the formulae of the following compounds:
(a) Ammonium carbonate (b) Barium sulphate (c) Calcium phosphate
Solution: The formulae of the given compounds are given below:
Compounds Ions Formula
(a) Ammonium carbonate NH 02 CO 2– (NH ) (CO ) o (NH ) CO
+
4 3 4 2 3 1 4 2 3
2+
(b) Barium sulphate Ba 02 SO 4 2– Ba (SO ) BaSO 4
2
4 2
(c) Calcium phosphate Ca 02 PO 4 3– Ca (PO ) o Ca (PO )
2+
3
4 2
3
4 2
EXAMPLE 1.4 Write the chemical formulae of the following:
(a) Magnesium chloride (b) Calcium oxide
(c) Copper nitrate (d) Aluminium chloride
(e) Calcium carbonate