Page 116 - Chemistry ICSE Class X
P. 116
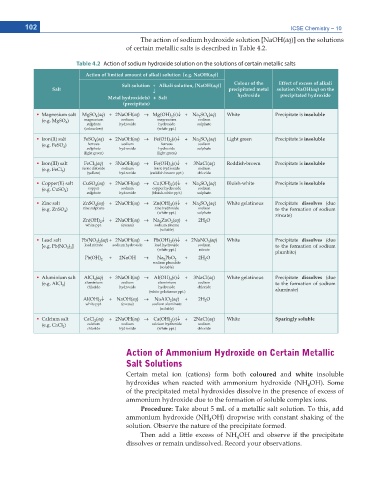
102 ICSE Chemistry – 10
The action of sodium hydroxide solution [NaOH(aq)] on the solutions
of certain metallic salts is described in Table 4.2.
Table 4.2 Action of sodium hydroxide solution on the solutions of certain metallic salts
Action of limited amount of alkali solution [e.g. NaOH(aq)]
Salt solution + Alkali solution, [NaOH(aq)] Colour of the Effect of excess of alkali
Salt p precipitated metal solution NaOH(aq) on the
Metal hydroxide(s) + Salt hydroxide precipitated hydroxide
(precipitate)
Ŗ Magnesium salt MgSO (aq) + 2NaOH(aq) o Mg(OH) (s)p + Na SO (aq) White Precipitate is insoluble
2
2
4
4
sodium
sodium
(e.g. MgSO ) magnesium hydroxide magnesium sulphate
4
hydroxide
sulphate
(colourless) (white ppt.)
Ŗ Iron(II) salt FeSO (aq) + 2NaOH(aq) o Fe(OH) (s)p + Na SO (aq) Light green Precipitate is insoluble
4
2
4
2
ferrous
sodium
ferrous
(e.g. FeSO ) sulphate hydroxide hydroxide sulphate
sodium
4
(light green) (light green)
Ŗ Iron(III) salt FeCl (aq) + 3NaOH(aq) o Fe(OH) (s)p + 3NaCl(aq) Reddish-brown Precipitate is insoluble
3
3
sodium
ferric hydroxide
sodium
(e.g. FeCl ) ferric chloride hydroxide (reddish brown ppt.) chloride
3
(yellow)
Ŗ Copper(II) salt CuSO (aq) + 2NaOH(aq) o Cu(OH) (s)p + Na SO (aq) Bluish-white Precipitate is insoluble
4
2
2
4
sodium
copper hydroxide
sodium
(e.g. CuSO ) sulphate hydroxide (bluish-white ppt.) sulphate
copper
4
Ŗ Zinc salt ZnSO (aq) + 2NaOH(aq) o Zn(OH) (s)p + Na SO (aq) White gelatinous Precipitate dissolves (due
4
2
2
4
(e.g. ZnSO ) zinc sulphate zinc hydroxide sulphate to the formation of sodium
sodium
4
(white ppt.)
Zn(OH) p + 2NaOH(aq) o Na ZnO (aq) + 2H O zincate)
2
2
2
2
white ppt. (excess) sodium zincate
(soluble)
Ŗ Lead salt Pb(NO ) (aq) + 2NaOH(aq) o Pb(OH) (s)p + 2NaNO (aq) White Precipitate dissolves (due
3 2
3
2
[e.g. Pb(NO ) ] lead nitrate sodium hydroxide lead hydroxide sodium to the formation of sodium
3 2
nitrate
(white ppt.)
plumbite)
Pb(OH) 2 + 2NaOH o Na PbO 2 + 2H O
2
2
sodium plumbite
(soluble)
Ŗ Aluminium salt AlCl (aq) + 3NaOH(aq) o Al(OH) (s)p + 3NaCl(aq) White gelatinous Precipitate dissolves (due
3
3
(e.g. AlCl ) aluminium sodium aluminium sodium to the formation of sodium
3
chloride hydroxide hydroxide chloride
(white gelatinous ppt.) aluminate)
Al(OH) p + NaOH(aq) o NaAlO (aq) + 2H O
2
3
2
white ppt. (excess) sodium aluminate
(soluble)
Ŗ Calcium salt CaCl (aq) + 2NaOH(aq) o Ca(OH) (s)p + 2NaCl(aq) White Sparingly soluble
2
2
sodium
calcium
sodium
(e.g. CaCl ) chloride hydroxide calcium hydroxide chloride
2
(white ppt.)
Action of Ammonium Hydroxide on Certain Metallic
Salt Solutions
Certain metal ion (cations) form both coloured and white insoluble
hydroxides when reacted with ammonium hydroxide (NH OH). Some
4
of the precipitated metal hydroxides dissolve in the presence of excess of
ammonium hydroxide due to the formation of soluble complex ions.
Procedure: Take about 5 mL of a metallic salt solution. To this, add
ammonium hydroxide (NH OH) dropwise with constant shaking of the
4
solution. Observe the nature of the precipitate formed.
Then add a little excess of NH OH and observe if the precipitate
4
dissolves or remain undissolved. Record your observations.