Page 115 - Chemistry ICSE Class X
P. 115
Analytical Chemistry 101
Table 4.1 List of ions forming colourless and coloured salts
Cations forming colourless salts Cations forming coloured salts Colour*
Name Formula Colour Name Formula Solid Solution
Ammonium NH + 4 Colourless Copper(II) or Cupric Cu 2+ Blue Blue
Sodium Na + Colourless Iron(II) or Ferrous Fe 2+ Light green Light green
Potassium K + Colourless Iron(III) or Ferric Fe 3+ Yellow to Brown Yellow
Calcium Ca 2+ Colourless Nickel(II) Ni 2+ Green Green
Magnesium Mg 2+ Colourless Chromium(III) Cr 3+ Bluish green to violet Bluish green
Aluminium Al 3+ Colourless Manganese(II) or Mn 2+ Light pink Light pink
Manganous
Lead Pb 2+ Colourless Cobalt(II) or Cobaltous Co 2+ Violet to pink Pink
Zinc Zn 2+ Colourless *These colours are for crystalline form of salts,
i.e., with water of crystallisation.
Anions forming colourless salts Anions forming coloured salts Colour
Name Formula Colour Name Formula Solid Solution
Carbonate CO 2– Colourless Permanganate MnO 4 – Purple with greenish Purple
3
lustre
Bicarbonate HCO 3 – Colourless Chromate CrO 4 2– Yellow Yellow
Sulphate SO 2– Colourless Dichromate Cr O 2– Orange Orange
2
7
4
Chloride Cl – Colourless
Acetate CH COO – Colourless
3
Note: +P OCP[ ECUGU VJG EQNQWT IGVU UNKIJVN[ OQFKſGF FGRGPFKPI WRQP VJG PCVWTG QH CPKQP
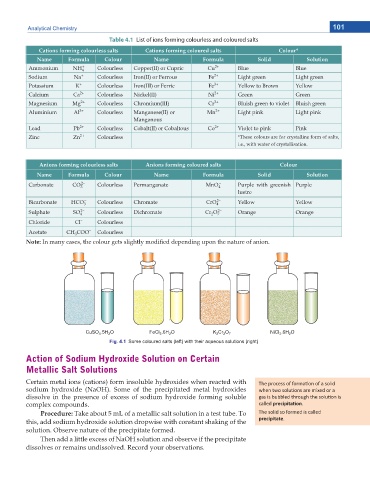
CuSO .5H O FeCl .6H O K Cr O 7 NiCl .6H O
2
2
4
2
2
2
2
3
Fig. 4.1 Some coloured salts (left) with their aqueous solutions (right)
Action of Sodium Hydroxide Solution on Certain
Metallic Salt Solutions
Certain metal ions (cations) form insoluble hydroxides when reacted with dŚĞ ƉƌŽĐĞƐƐ ŽĨ ĨŽƌŵĂƟŽŶ ŽĨ Ă ƐŽůŝĚ
sodium hydroxide (NaOH). Some of the precipitated metal hydroxides ǁŚĞŶ ƚǁŽ ƐŽůƵƟŽŶƐ ĂƌĞ ŵŝdžĞĚ Žƌ Ă
dissolve in the presence of excess of sodium hydroxide forming soluble ŐĂƐ ŝƐ ďƵďďůĞĚ ƚŚƌŽƵŐŚ ƚŚĞ ƐŽůƵƟŽŶ ŝƐ
complex compounds. ĐĂůůĞĚ ƉƌĞĐŝƉŝƚĂƟŽŶ.
Procedure: Take about 5 mL of a metallic salt solution in a test tube. To dŚĞ ƐŽůŝĚ ƐŽ ĨŽƌŵĞĚ ŝƐ ĐĂůůĞĚ
this, add sodium hydroxide solution dropwise with constant shaking of the precipitate.
solution. Observe nature of the precipitate formed.
Then add a little excess of NaOH solution and observe if the precipitate
dissolves or remains undissolved. Record your observations.