Page 117 - Chemistry ICSE Class X
P. 117
Analytical Chemistry 103
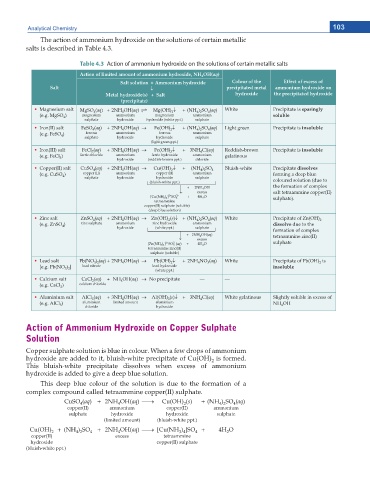
The action of ammonium hydroxide on the solutions of certain metallic
salts is described in Table 4.3.
Table 4.3 Action of ammonium hydroxide on the solutions of certain metallic salts
Action of limited amount of ammonium hydroxide, NH OH(aq)
4
Salt solution + Ammonium hydroxide Colour of the Effect of excess of
Salt p precipitated metal ammonium hydroxide on
Metal hydroxide(s) + Salt hydroxide the precipitated hydroxide
(precipitate)
Ŗ Magnesium salt MgSO 4 (aq) + 2NH 4 OH(aq) U Mg(OH) 2 p + (NH 4 ) 2 SO 4 (aq) White Precipitate is sparingly
(e.g. MgSO ) magnesium ammonium magnesium ammonium soluble
4
sulphate hydroxide hydroxide (white ppt.) sulphate
Ŗ Iron(II) salt FeSO (aq) + 2NH OH(aq) o Fe(OH) p + (NH ) SO (aq) Light green Precipitate is insoluble
4
2
4
4
4 2
(e.g. FeSO ) ferrous ammonium ferrous ammonium
4
sulphate hydroxide hydroxide sulphate
(light green ppt.)
Ŗ Iron(III) salt FeCl (aq) + 3NH OH(aq) o Fe(OH) p + 3NH Cl(aq) Reddish-brown Precipitate is insoluble
3
3
4
4
ferric hydroxide
(e.g. FeCl ) ferric chloride ammonium (reddish-brown ppt.) ammonium gelatinous
3
hydroxide
chloride
Ŗ Copper(II) salt CuSO 4 (aq) + 2NH 4 OH(aq) o Cu(OH) 2 p + (NH 4 ) 2 SO 4 Bluish-white Precipitate dissolves
) copper (II) ammonium copper (II) ammonium forming a deep blue
(e.g. CuSO 4
sulphate hydroxide hydroxide sulphate
(bluish-white ppt.) coloured solution (due to
+ 2NH 4 OH the formation of complex
excess salt tetraammine copper(II)
o
2+ 2– + 4H 2 O
[Cu(NH 3 ) 4 ] SO 4 sulphate).
tetraammine
copper(II) sulphate (soluble)
(deep blue solution)
Ŗ Zinc salt ZnSO 4 (aq) + 2NH OH(aq) o Zn(OH) (s)p + (NH ) SO (aq) White Precipitate of Zn(OH)
4
4 2
2
2
4
(e.g. ZnSO ) zinc sulphate ammonium zinc hydroxide ammonium dissolve due to the
4
hydroxide
sulphate
(white ppt.)
+ 2NH 4 OH(aq) formation of complex
tetraammine zinc(II)
excess
o
2–
2+
[Zn(NH 3 ) 4 ] SO 4 (aq) + 4H 2 O sulphate
tetraammine zinc(II)
sulphate (soluble)
Ŗ Lead salt Pb(NO 3 2 4 Pb(OH) p + 2NH NO (aq) White Precipitate of Pb(OH) is
) (aq) + 2NH OH(aq) o
2
2
4
3
[e.g. Pb(NO ) ] lead nitrate lead hydroxide insoluble
3 2
(white ppt.)
Ŗ Calcium salt CaCl 2 (aq) + NH OH(aq) o No precipitate — —
4
(e.g. CaCl ) calcium chloride
2
Ŗ Aluminium salt AlCl (aq) + 3NH OH(aq) o Al(OH) (s)p + 3NH Cl(aq) White gelatinous Slightly soluble in excess of
4
3
3
4
(e.g. AlCl ) aluminium limited amount aluminium NH OH
3
4
chloride
hydroxide
Action of Ammonium Hydroxide on Copper Sulphate
Solution
Copper sulphate solution is blue in colour. When a few drops of ammonium
hydroxide are added to it, bluish-white precipitate of Cu(OH) is formed.
2
This bluish-white precipitate dissolves when excess of ammonium
hydroxide is added to give a deep blue solution.
This deep blue colour of the solution is due to the formation of a
complex compound called tetraammine copper(II) sulphate.
CuSO (aq) + 2NH OH(aq) o Cu(OH) (s) + (NH ) SO (aq)
4 2
4
4
2
4
copper(II) ammonium copper(II) ammonium
sulphate hydroxide hydroxide sulphate
(limited amount) (bluish-white ppt.)
Cu(OH) + (NH ) SO + 2NH OH(aq) o [Cu(NH ) ]SO + 4H O
2
4 2
2
4
4
4
3 4
copper(II) excess tetraammine
hydroxide copper(II) sulphate
(bluish-white ppt.)