Page 120 - Chemistry ICSE Class X
P. 120
106 ICSE Chemistry – 10
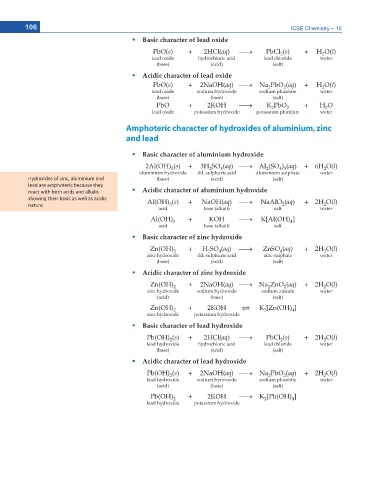
Basic character of lead oxide
PbO(s) + 2HCl(aq) o PbCl (s) + H O(l)
2
2
lead oxide hydrochloric acid lead chloride water
(base) (acid) (salt)
Acidic character of lead oxide
PbO(s) + 2NaOH(aq) o Na PbO (aq) + H O(l)
2
2
2
lead oxide sodium hydroxide sodium plumbite water
(base) (base) (salt)
PbO + 2KOH o K PbO 2 + H O
2
2
lead oxide potassium hydroxide potassium plumbite water
Amphoteric character of hydroxides of aluminium, zinc
and lead
Basic character of aluminium hydroxide
2Al(OH) (s) + 3H SO (aq) o Al (SO ) (aq) + 6H O(l)
4 3
2
4
2
3
2
aluminium hydroxide dil. sulphuric acid aluminium sulphate water
,LJĚƌŽdžŝĚĞƐ ŽĨ njŝŶĐ͕ ĂůƵŵŝŶŝƵŵ ĂŶĚ (base) (acid) (salt)
ůĞĂĚ ĂƌĞ ĂŵƉŚŽƚĞƌŝĐ ďĞĐĂƵƐĞ ƚŚĞLJ
ƌĞĂĐƚ ǁŝƚŚ ďŽƚŚ ĂĐŝĚƐ ĂŶĚ ĂůŬĂůŝƐ Acidic character of aluminium hydroxide
ƐŚŽǁŝŶŐ ƚŚĞŝƌ ďĂƐŝĐ ĂƐ ǁĞůů ĂƐ ĂĐŝĚŝĐ Al(OH) (s) + NaOH(aq) o NaAlO (aq) + 2H O(l)
ŶĂƚƵƌĞ͘ 3 2 2
acid base (alkali) salt water
Al(OH) 3 + KOH o K[Al(OH) ]
4
acid base (alkali) salt
Basic character of zinc hydroxide
Zn(OH) 2 + H SO (aq) o ZnSO (aq) + 2H O(l)
2
4
2
4
zinc hydroxide dil. sulphuric acid zinc sulphate water
(base) (acid) (salt)
Acidic character of zinc hydroxide
Zn(OH) 2 + 2NaOH(aq) o Na ZnO (aq) + 2H O(l)
2
2
2
zinc hydroxide sodium hydroxide sodium zincate water
(acid) (base) (salt)
Zn(OH) 2 + 2KOH U K [Zn(OH) ]
2
4
zinc hydroxide potassium hydroxide
Basic character of lead hydroxide
Pb(OH) (s) + 2HCl(aq) o PbCl (s) + 2H O(l)
2
2
2
lead hydroxide hydrochloric acid lead chloride water
(base) (acid) (salt)
Acidic character of lead hydroxide
Pb(OH) (s) + 2NaOH(aq) o Na PbO (aq) + 2H O(l)
2
2
2
2
lead hydroxide sodium hydroxide sodium plumbite water
(acid) (base) (salt)
Pb(OH) 2 + 2KOH o K [Pb(OH) ]
2
4
lead hydroxide potassium hydroxide