Page 144 - Chemistry ICSE Class X
P. 144
130 ICSE Chemistry – 10
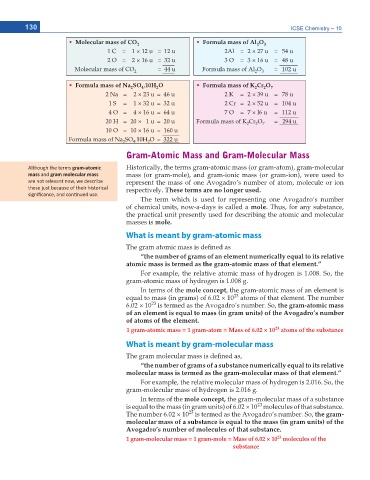
Ŗ Molecular mass of CO 2 Ŗ Formula mass of Al O 3
2
1 C = 1 × 12 u = 12 u 2Al = 2 × 27 u = 54 u
2 O = 2 × 16 u = 32 u 3 O = 3 × 16 u = 48 u
Molecular mass of CO 2 = 44 u Formula mass of Al O 3 = 102 u
2
Ŗ Formula mass of Na SO .10H O Ŗ Formula mass of K Cr O 7
2
4
2
2
2
2 Na = 2 × 23 u = 46 u 2 K = 2 × 39 u = 78 u
1 S = 1 × 32 u = 32 u 2 Cr = 2 × 52 u = 104 u
4 O = 4 × 16 u = 64 u 7 O = 7 × l6 u = 112 u
20 H = 20 × 1 u = 20 u Formula mass of K Cr O = 294 u
2
2
7
10 O = 10 × 16 u = 160 u
Formula mass of Na SO .10H O = 322 u
2
2
4
Gram-Atomic Mass and Gram-Molecular Mass
ůƚŚŽƵŐŚ ƚŚĞ ƚĞƌŵƐ gram-atomic Historically, the terms gram-atomic mass (or gram-atom), gram-molecular
mass ĂŶĚ gram molecular mass mass (or gram-mole), and gram-ionic mass (or gram-ion), were used to
ĂƌĞ ŶŽƚ ƌĞůĞǀĂŶƚ ŶŽǁ͕ ǁĞ ĚĞƐĐƌŝďĞ represent the mass of one Avogadro’s number of atom, molecule or ion
ƚŚĞƐĞ ũƵƐƚ ďĞĐĂƵƐĞ ŽĨ ƚŚĞŝƌ ŚŝƐƚŽƌŝĐĂů respectively. These terms are no longer used.
ƐŝŐŶŝĮĐĂŶĐĞ͕ ĂŶĚ ĐŽŶƟŶƵĞĚ ƵƐĞ͘
The term which is used for representing one Avogadro‘s number
of chemical units, now-a-days is called a mole. Thus, for any substance,
the practical unit presently used for describing the atomic and molecular
masses is mole.
What is meant by gram-atomic mass
6JG ITCO CVQOKE OCUU KU FGſPGF CU
“the number of grams of an element numerically equal to its relative
atomic mass is termed as the gram-atomic mass of that element.”
For example, the relative atomic mass of hydrogen is 1.008. So, the
gram-atomic mass of hydrogen is 1.008 g.
In terms of the mole concept, the gram-atomic mass of an element is
23
equal to mass (in grams) of 6.02 × 10 atoms of that element. The number
23
6.02 × 10 is termed as the Avogadro’s number. So, the gram-atomic mass
of an element is equal to mass (in gram units) of the Avogadro’s number
of atoms of the element.
23
1 gram-atomic mass = 1 gram-atom = Mass of 6.02 × 10 atoms of the substance
What is meant by gram-molecular mass
6JG ITCO OQNGEWNCT OCUU KU FGſPGF CU
“the number of grams of a substance numerically equal to its relative
molecular mass is termed as the gram-molecular mass of that element.”
For example, the relative molecular mass of hydrogen is 2.016. So, the
gram-molecular mass of hydrogen is 2.016 g.
In terms of the mole concept, the gram-molecular mass of a substance
23
is equal to the mass (in gram units) of 6.02 × 10 molecules of that substance.
23
The number 6.02 × 10 is termed as the Avogadro’s number. So, the gram-
molecular mass of a substance is equal to the mass (in gram units) of the
Avogadro’s number of molecules of that substance.
23
1 gram-molecular mass = 1 gram-mole = Mass of 6.02 × 10 molecules of the
substance