Page 193 - Chemistry ICSE Class X
P. 193
Metallurgy 179
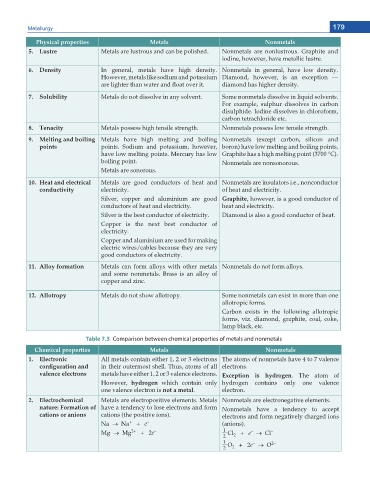
Physical properties Metals Nonmetals
5. Lustre Metals are lustrous and can be polished. Nonmetals are nonlustrous. Graphite and
iodine, however, have metallic lustre.
6. Density In general, metals have high density. Nonmetals in general, have low density.
However, metals like sodium and potassium Diamond, however, is an exception —
CTG NKIJVGT VJCP YCVGT CPF ƀQCV QXGT KV diamond has higher density.
7. Solubility Metals do not dissolve in any solvent. Some nonmetals dissolve in liquid solvents.
For example, sulphur dissolves in carbon
disulphide. Iodine dissolves in chloroform,
carbon tetrachloride etc.
8. Tenacity Metals possess high tensile strength. Nonmetals possess low tensile strength.
9. Melting and boiling Metals have high melting and boiling Nonmetals (except carbon, silicon and
points points. Sodium and potassium, however, boron) have low melting and boiling points,
have low melting points. Mercury has low Graphite has a high melting point (3700 °C).
boiling point. Nonmetals are nonsonorous.
Metals are sonorous.
10. Heat and electrical Metals are good conductors of heat and Nonmetals are insulators i.e., nonconductor
conductivity electricity. of heat and electricity.
Silver, copper and aluminium are good Graphite, however, is a good conductor of
conductors of heat and electricity. heat and electricity.
Silver is the best conductor of electricity. Diamond is also a good conductor of heat.
Copper is the next best conductor of
electricity.
Copper and aluminium are used for making
electric wires/cables because they are very
good conductors of electricity.
11. Alloy formation Metals can form alloys with other metals Nonmetals do not form alloys.
and some nonmetals. Brass is an alloy of
copper and zinc.
12. Allotropy Metals do not show allotropy. Some nonmetals can exist in more than one
allotropic forms.
Carbon exists in the following allotropic
forms, viz. diamond, graphite, coal, coke,
lamp black, etc.
Table 7.3 Comparison between chemical properties of metals and nonmetals
Chemical properties Metals Nonmetals
1. Electronic All metals contain either 1, 2 or 3 electrons The atoms of nonmetals have 4 to 7 valence
EQPſIWTCVKQP CPF in their outermost shell. Thus, atoms of all electrons.
valence electrons metals have either 1, 2 or 3 valence electrons. Exception is hydrogen. The atom of
However, hydrogen which contain only hydrogen contains only one valence
one valence electron is not a metal. electron.
2. Electrochemical Metals are electropositive elements. Metals Nonmetals are electronegative elements.
nature: Formation of have a tendency to lose electrons and form Nonmetals have a tendency to accept
cations or anions cations (the positive ions). electrons and form negatively charged ions
+
Na o Na + e – (anions).
–
2+
Mg o Mg + 2e – 1 Cl + e o Cl –
2 2
1 O + 2e o O 2–
–
2 2