Page 29 - Chemistry ICSE Class X
P. 29
Periodic Properties and Variations of Properties (Physical and Chemical) 17
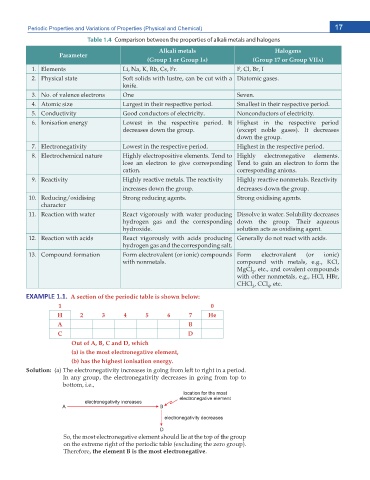
Table 1.4 Comparison between the properties of alkali metals and halogens
Alkali metals Halogens
Parameter
(Group 1 or Group IA) (Group 17 or Group VIIA)
Elements .K 0C - 4D %U (T F, Cl, Br, I
Physical state Soft solids with lustre, can be cut with a &KCVQOKE ICUGU
MPKHG
0Q QH XCNGPEG GNGEVTQPU One 5GXGP
Atomic size .CTIGUV KP VJGKT TGURGEVKXG RGTKQF 5OCNNGUV KP VJGKT TGURGEVKXG RGTKQF
Conductivity )QQF EQPFWEVQTU QH GNGEVTKEKV[ 0QPEQPFWEVQTU QH GNGEVTKEKV[
Ionisation energy .QYGUV KP VJG TGURGEVKXG RGTKQF +V Highest in the respective period
FGETGCUGU FQYP VJG ITQWR GZEGRV PQDNG ICUGU +V FGETGCUGU
FQYP VJG ITQWR
Electronegativity .QYGUV KP VJG TGURGEVKXG RGTKQF *KIJGUV KP VJG TGURGEVKXG RGTKQF
Electrochemical nature *KIJN[ GNGEVTQRQUKVKXG GNGOGPVU 6GPF VQ *KIJN[ GNGEVTQPGICVKXG GNGOGPVU
lose an electron to give corresponding Tend to gain an electron to form the
ECVKQP EQTTGURQPFKPI CPKQPU
Reactivity *KIJN[ TGCEVKXG OGVCNU 6JG TGCEVKXKV[ *KIJN[ TGCEVKXG PQPOGVCNU 4GCEVKXKV[
KPETGCUGU FQYP VJG ITQWR FGETGCUGU FQYP VJG ITQWR
4GFWEKPI QZKFKUKPI 5VTQPI TGFWEKPI CIGPVU 5VTQPI QZKFKUKPI CIGPVU
character
Reaction with water React vigorously with water producing &KUUQNXG KP YCVGT 5QNWDKNKV[ FGETGCUGU
hydrogen gas and the corresponding FQYP VJG ITQWR 6JGKT CSWGQWU
J[FTQZKFG UQNWVKQP CEVU CU QZKFKUKPI CIGPV
Reaction with acids React vigorously with acids producing )GPGTCNN[ FQ PQV TGCEV YKVJ CEKFU
J[FTQIGP ICU CPF VJG EQTTGURQPFKPI UCNV
Compound formation Form electrovalent (or ionic) compounds Form electrovalent (or ionic)
YKVJ PQPOGVCNU EQORQWPF YKVJ OGVCNU G I -%N
MgCl GVE CPF EQXCNGPV EQORQWPFU
2
YKVJ QVJGT PQPOGVCNU G I *%N *$T
CHCl , CCl GVE
3 4
y DW> ϭ͘ϭ͘ A section of the periodic table is shown below:
1 0
H 2 3 4 5 6 7 He
A B
C D
Out of A, B, C and D, which
(a) is the most electronegative element,
(b) has the highest ionisation energy.
Solution: C 6JG GNGEVTQPGICVKXKV[ KPETGCUGU KP IQKPI HTQO NGHV VQ TKIJV KP C RGTKQF
In any group, the electronegativity decreases in going from top to
DQVVQO K G
So, the most electronegative element should lie at the top of the group
QP VJG GZVTGOG TKIJV QH VJG RGTKQFKE VCDNG GZENWFKPI VJG \GTQ ITQWR
Therefore, the element B is the most electronegative