Page 109 - Chemistry ICSE Class IX
P. 109
Atomic Structure and Chemical Bonding 97
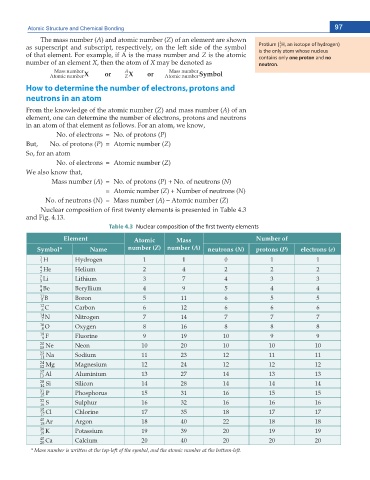
The mass number (A) and atomic number (Z) of an element are shown ϭ
as superscript and subscript, respectively, on the left side of the symbol WƌŽƟƵŵ ; ,͕ ĂŶ ŝƐŽƚŽƉĞ ŽĨ ŚLJĚƌŽŐĞŶͿ
ϭ
of that element. For example, if A is the mass number and Z is the atomic ŝƐ ƚŚĞ ŽŶůLJ ĂƚŽŵ ǁŚŽƐĞ ŶƵĐůĞƵƐ
ĐŽŶƚĂŝŶƐ ŽŶůLJ one proton ĂŶĚ no
number of an element X, then the atom of X may be denoted as neutron.
How to determine the number of electrons, protons and
neutrons in an atom
From the knowledge of the atomic number (Z) and mass number (A) of an
element, one can determine the number of electrons, protons and neutrons
in an atom of that element as follows. For an atom, we know,
No. of electrons = No. of protons (P)
But, No. of protons (P) = Atomic number (Z)
So, for an atom
No. of electrons = Atomic number (Z)
We also know that,
Mass number (A) = No. of protons (P) + No. of neutrons (N)
= Atomic number (Z) + Number of neutrons (N)
No. of neutrons (N) = Mass number (A) – Atomic number (Z)
Nuclear composition of rst twenty elements is presented in Table 4.3
and Fig. 4.13.
Table 4.3 Nuclear composition of the first twenty elements
Element Atomic Mass Number of
Symbol* Name number (Z) number (A) neutrons (N) protons (P) electrons (e)
1 H Hydrogen 1 1 0 1 1
1
4 He Helium 2 4 2 2 2
2
7 Li Lithium 3 7 4 3 3
3
9 Be Beryllium 4 9 5 4 4
4
11 B Boron 5 11 6 5 5
5
12 C Carbon 6 12 6 6 6
6
14 N Nitrogen 7 14 7 7 7
7
16 O Oxygen 8 16 8 8 8
8
19 F Fluorine 9 19 10 9 9
9
20 Ne Neon 10 20 10 10 10
10
23 Na Sodium 11 23 12 11 11
11
24 Mg Magnesium 12 24 12 12 12
12
27 Al Aluminium 13 27 14 13 13
13
28 Si Silicon 14 28 14 14 14
14
31 P Phosphorus 15 31 16 15 15
15
32 S Sulphur 16 32 16 16 16
16
35 Cl Chlorine 17 35 18 17 17
17
40 Ar Argon 18 40 22 18 18
18
39 K Potassium 19 39 20 19 19
19
40 Ca Calcium 20 40 20 20 20
20
* Mass number is written at the top-left of the symbol, and the atomic number at the bottom-left.