Page 110 - Chemistry ICSE Class IX
P. 110
98 ICSE Chemistry – 9
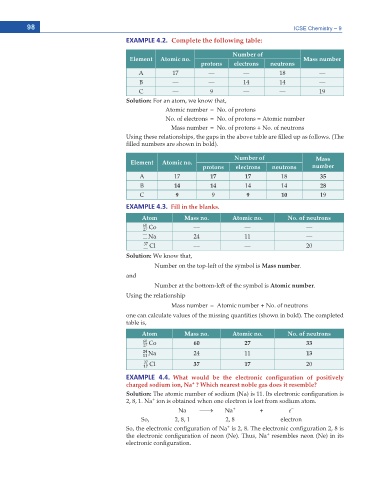
EXAMPLE 4.2.ഩComplete the following table:
Number of
Element Atomic no. Mass number
protons electrons neutrons
A 17 –– –– 18 ––
B –– –– 14 14 ––
C –– 9 –– –– 19
Solution: For an atom, we know that,
Atomic number = No. of protons
No. of electrons = No. of protons = Atomic number
Mass number = No. of protons + No. of neutrons
Using these relationships, the gaps in the above table are lled up as follows. (The
lled numbers are shown in bold).
Number of Mass
Element Atomic no.
protons electrons neutrons number
A 17 17 17 18 35
B 14 14 14 14 28
C 9 9 9 10 19
EXAMPLE 4.3. Fill in the blanks.
Atom Mass no. Atomic no. No. of neutrons
60 Co –– –– ––
27
–– Na 24 11 ––
––
37 Cl –– –– 20
––
Solution: We know that,
Number on the top-left of the symbol is Mass number.
and
Number at the bottom-left of the symbol is Atomic number.
Using the relationship
Mass number = Atomic number + No. of neutrons
one can calculate values of the missing quantities (shown in bold). The completed
table is,
Atom Mass no. Atomic no. No. of neutrons
60 Co 60 27 33
27
24 Na 24 11 13
11
37 Cl 37 17 20
17
EXAMPLE 4.4. What would be the electronic configuration of positively
+
charged sodium ion, Na ? Which nearest noble gas does it resemble?
Solution: The atomic number of sodium (Na) is 11. Its electronic con guration is
+
2, 8, 1. Na ion is obtained when one electron is lost from sodium atom.
–
Na o Na + + e
So, 2, 8, 1 2, 8 electron
+
So, the electronic con guration of Na is 2, 8. The electronic con guration 2, 8 is
+
the electronic con guration of neon (Ne). Thus, Na resembles neon (Ne) in its
electronic con guration.