Page 116 - Chemistry ICSE Class IX
P. 116
104 ICSE Chemistry – 9
Thus,
16
Percentage of X = 90%
8
18
and Percentage of X = (100 – 90)% = 10%
8
Chemical Reactivity and Electronic Configuration
Atoms of different elements have different electronic con gurations.
Different elements also show different chemical properties. Here, we
describe relationship between the electronic con guration of an element
and its chemical behaviour.
Why do atoms combine
Atoms combine with each other to form various compounds. The smallest
unit of a substance which can exist independently is called a molecule. So,
atoms combine with each other to form molecules.
What makes these atoms combine to form molecules of various
compounds? What is the nature of forces which keep the atoms together
in a molecule?
The chemical behaviour of any element depends upon the position of
the element in the periodic table. The position of an element in the periodic
table is related to its electronic con guration. So, the chemical reactivity of
CP GNGOGPV FGRGPFU WRQP KVU GNGEVTQPKE EQPſIWTCVKQP i.e., the chemical
reactivity of an element depends upon the distribution of electrons in its
atom.
It has been found that the atoms having a total of 2, 10, 18, 36, 54 and
dŚĞ ŽƵƚĞƌŵŽƐƚ Žƌďŝƚ ŝŶ ƚŚĞ ĂƚŽŵ ŽĨ 86 electrons are the most stable, i.e., such atoms do not show any chemical
ĞĂĐŚ ŶŽďůĞ ŐĂƐ ŚĂƐ ĞŝŐŚƚ ĞůĞĐƚƌŽŶƐ͕
ĞdžĐĞƉƚ ŝŶ ƚŚĞ ĐĂƐĞ ŽĨ ŚĞůŝƵŵ reactivity. These electronic con gurations are those of the noble gases, viz.,
ǁŚŝĐŚ ŚĂƐ ŽŶůLJ ƚǁŽ ĞůĞĐƚƌŽŶƐ ŝŶ ŝƚƐ Helium, Neon, Argon, Krypton, Xenon and Radon, respectively. These
ŽƵƚĞƌŵŽƐƚ Žƌďŝƚ͘ elements, called noble gases, do not show any chemical reactivity. Thus,
the noble gases do not form compounds either among themselves or with
other elements. Xenon, however, forms uorides and oxy uorides under
controlled conditions. This nonreactivity of noble gases is due to their
UVCDNG GNGEVTQPKE EQPſIWTCVKQPU. The electronic con gurations of these
elements are given in Table 4.5.
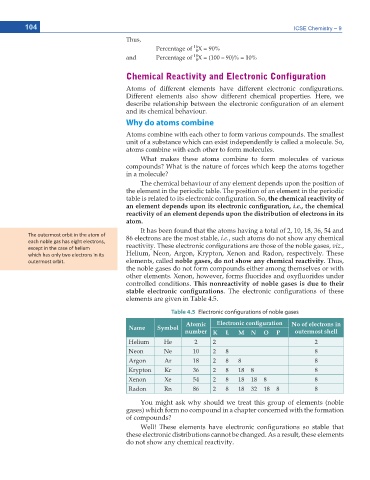
Table 4.5 Electronic configurations of noble gases
Atomic 'NGEVTQPKE EQPſIWTCVKQP No of electrons in
Name Symbol
number K L M N O P outermost shell
Helium He 2 2 2
Neon Ne 10 2 8 8
Argon Ar 18 2 8 8 8
Krypton Kr 36 2 8 18 8 8
Xenon Xe 54 2 8 18 18 8 8
Radon Rn 86 2 8 18 32 18 8 8
You might ask why should we treat this group of elements (noble
gases) which form no compound in a chapter concerned with the formation
of compounds?
Well These elements have electronic con gurations so stable that
these electronic distributions cannot be changed. As a result, these elements
do not show any chemical reactivity.