Page 118 - Chemistry ICSE Class IX
P. 118
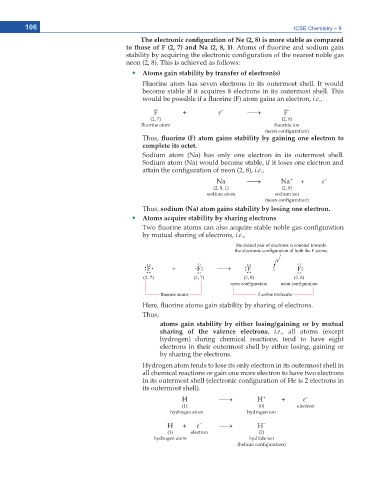
106 ICSE Chemistry – 9
6JG GNGEVTQPKE EQPſIWTCVKQP QH 0G KU OQTG UVCDNG CU EQORCTGF
to those of F (2, 7) and Na (2, 8, 1). Atoms of uorine and sodium gain
stability by acquiring the electronic con guration of the nearest noble gas
neon (2, 8). This is achieved as follows:
Atoms gain stability by transfer of electron(s)
Fluorine atom has seven electrons in its outermost shell. It would
become stable if it acquires 8 electrons in its outermost shell. This
would be possible if a uorine (F) atom gains an electron, i.e.,
–
F + e o F –
(2, 7) (2, 8)
uorine atom uoride ion
(neon con guration)
Thus, ƀWQTKPG ( CVQO ICKPU UVCDKNKV[ D[ ICKPKPI QPG GNGEVTQP VQ
complete its octet.
Sodium atom (Na) has only one electron in its outermost shell.
Sodium atom (Na) would become stable, if it loses one electron and
attain the con guration of neon (2, 8), i.e.,
+
Na o Na + e –
(2, 8, 1) (2, 8)
sodium atom sodium ion
(neon con guration)
Thus, sodium (Na) atom gains stability by losing one electron.
Atoms acquire stability by sharing electrons
Two uorine atoms can also acquire stable noble gas con guration
by mutual sharing of electrons, i.e.,
Here, uorine atoms gain stability by sharing of electrons.
Thus,
atoms gain stability by either losing/gaining or by mutual
sharing of the valence electrons, i.e., all atoms (except
hydrogen) during chemical reactions, tend to have eight
electrons in their outermost shell by either losing, gaining or
by sharing the electrons.
Hydrogen atom tends to lose its only electron in its outermost shell in
all chemical reactions or gain one more electron to have two electrons
in its outermost shell (electronic con guration of He is 2 electrons in
its outermost shell).
H o H + + e –
(1) (0) electron
hydrogen atom hydrogen ion
–
H + e o H –
(1) electron (2)
hydrogen atom hydride ion
(helium con guration)